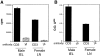An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes - PubMed (original) (raw)
An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes
D Cruz et al. J Exp Med. 1998.
Abstract
The differentiation of intestinal intraepithelial lymphocytes (IEL) remains controversial, which may be due in part to the phenotypic complexity of these T cells. We have investigated here the development of IEL in mice on the recombination activating gene (RAG)-2(-/-) background which express a T cell antigen receptor (TCR) transgene specific for an H-Y peptide presented by Db (H-Y/Db x RAG-2(-) mice). In contrast to the thymus, the small intestine in female H-Y/Db x RAG-2(-) mice is severely deficient in the number of IEL; TCR transgene+ CD8alphaalpha and CD8alphabeta are virtually absent. This is similar to the number and phenotype of IEL in transgenic mice that do not express the Db class I molecule, and which therefore fail positive selection. Paradoxically, in male mice, the small intestine contains large numbers of TCR+ IEL that express high levels of CD8alphaalpha homodimers. The IEL isolated from male mice are functional, as they respond upon TCR cross-linking, although they are not autoreactive to stimulator cells from male mice. We hypothesize that the H-Y/Db TCR fails to undergo selection in IEL of female mice due to the reduced avidity of the TCR for major histocompatibility complex peptide in conjunction with the CD8alphaalpha homodimers expressed by many cells in this lineage. By contrast, this reduced TCR/CD8alphaalpha avidity may permit positive rather than negative selection of this TCR in male mice. Therefore, the data presented provide conclusive evidence that a TCR which is positively selected in the thymus will not necessarily be selected in IEL, and furthermore, that the expression of a distinct CD8 isoform by IEL may be a critical determinant of the differential pattern of selection of these T cells.
Figures
Figure 1
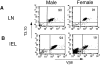
T cells from male and female H-Y/Db × RAG-2− mice express only the transgenic TCR. Male and female lymphocytes isolated from LNs (A) and IEL (B) were stained with the T3.70 mAb followed by anti–mouse IgG1-FITC antibody. Cells were then stained with anti– Vβ8-PE. Data from 15-wk-old mice are shown, representative of all such mice ages 8–20 wk analyzed. The percentage of cells positive for both the TCR transgenes is indicated in the upper right quadrant.
Figure 2
Opposite patterns of T cell selection are observed in the thymus and intestine of H-Y/Db transgenic × RAG-2− mice. Thymocytes and small intestine IEL were stained for TCR transgene expression with either Vβ8-PE, or Vβ8-biotin followed by streptavidin-tricolor. The number of TCR transgene+ cells was calculated by multiplying the total number of lymphocytes by the percentage of Vβ8+ cells as determined by flow cytometry analysis. Each symbol represents the analysis of cells from a single mouse. Values are as follows: female thymus (n = 3), 3.4 × 108 ± 0.03; male thymus (n = 7), 4.0 × 106 ± 0.9; female small intestine (n = 6) 7.7 × 104 ± 1.5; and male small intestine (n = 9), 1.3 × 107 ± 0.3.
Figure 3
CD8αα+ and CD8αβ+ IEL develop more efficiently in male than in female H-Y/Db transgenic mice. Three-color flow cytometry analysis was used to determine coreceptor expression by TCR+ cells in small intestine IEL preparations. (A) IEL from male transgenic mice express predominantly CD8αα homodimers, whereas the majority of female IEL are DN. The analysis of CD8 coreceptor expression is shown for TCR transgene+ (Vβ8+) cells from individual mice. Representative data from one of many different experiments are shown. (B) The TCR+ IEL that express CD8β in the females do not coexpress the αE integrin. The analysis of CD8β and αE expression is shown for Vβ8+ cells from individual mice. Representative data from one of two mice analyzed in this way.
Figure 3
CD8αα+ and CD8αβ+ IEL develop more efficiently in male than in female H-Y/Db transgenic mice. Three-color flow cytometry analysis was used to determine coreceptor expression by TCR+ cells in small intestine IEL preparations. (A) IEL from male transgenic mice express predominantly CD8αα homodimers, whereas the majority of female IEL are DN. The analysis of CD8 coreceptor expression is shown for TCR transgene+ (Vβ8+) cells from individual mice. Representative data from one of many different experiments are shown. (B) The TCR+ IEL that express CD8β in the females do not coexpress the αE integrin. The analysis of CD8β and αE expression is shown for Vβ8+ cells from individual mice. Representative data from one of two mice analyzed in this way.
Figure 4
Phenotype of small intestine IEL in TCR transgenic mice that lack expression of the positively selecting class I molecule. (A) IEL from an individual H-Y/Db × RAG-2− × TAP-1− 25-wk-old male mouse. (B) IEL from an individual H-Y/Db × RAG-2− × TAP-1− 25-wk-old female mouse. (C) IEL from an individual 18-wk-old Dd+ H-Y/Db × RAG-2− male mouse. Isolated IEL were stained with anti-CD8α, anti-CD8β, and anti-Vβ8 mAbs, and the two-color dot plots for CD8α and Vβ8 expression are displayed. There were no cells positive for CD8β staining in these nonselecting TCR transgenic mice. Data from these mice, which are slightly older than the average, were collected on a different day, and were not used in the compilation of TAP-1−/− mice in Table 1.
Figure 5
Levels of CD8 coreceptor expression by T cells from H-Y/ Db × RAG-2− transgenic mice. (A) CD8α staining. Cells obtained from the indicated sources from individual mice were stained for Vβ8-biotin and CD8α-PE, and the single-color CD8 histogram is shown for the Vβ8+ cells. (B) CD8β staining, determined with a CD8β-PE mAb, for the same individuals as described above for CD8α. Representative data from one of many experiments. MF, Mean fluorescence.
Figure 6
CD4+ IEL are absent in H-Y/Db × RAG-2− mice. Total IEL, with the lymphocyte gate set according to light scatter parameters, from individual male and female mice were stained with anti-CD4–FITC and anti–Vβ8-biotin, followed by streptavidin-tricolor. Representative data from one of many experiments are presented.
Figure 7
The CD8αα IEL subset from male H-Y/Db × RAG-2− mice can respond to CD3 cross-linking. CD8αα IEL were highly purified by cell sorting as described in Materials and Methods from a pool of two mice. Small intestine IEL from male mice, or total lymphocytes isolated from LNs of female H-Y/Db transgenic mice, were cultured in plates that had been coated either with 5 μg/ml of anti-CD3 mAb (2C11) or with a control hamster mAb (anti-γδ clone UC7) as described in Materials and Methods. Data are from triplicate wells in each case, with the SE of the mean indicated. (A) Proliferation assay. Cells were cultured for 48 h, pulsed with [3H]thymidine, and harvested 24 h later. (B) IFN-γ release. Cells were cultured for 48 h as described above, and then supernatants were assayed for cytokine release by ELISA as detailed in Materials and Methods.
Figure 8
A model for the opposite pattern of selection of the H-Y/Db TCR in thymocytes and IEL. Top, Hypothetical plot of thymocyte number versus TCR avidity for self-MHC in a normal mouse. Most cells are ignored and undergo programmed cell death as their TCRs fall into the no selection avidity range (open area), while only a minority fall into the range for positive selection (solid gray area). In the thymus of H-Y/Db × RAG-2− mice (middle), the avidity range of the monoclonal TCR population that expresses CD8αβ is very narrow, and falls into the positive selection range for females and the high avidity negative selection range for males (diagonal stripes). By contrast, in CD8αα+ IEL (bottom), the overall avidity of the TCR interaction is shifted to the left because of the lower avidity of the homodimeric form of CD8. This places the H-Y/Db TCR in female mice in the no selection range, and in male mice in the positive selection range.
Similar articles
- Late postnatal expansion of self-reactive CD8alphaalpha+ intestinal intraepithelial lymphocytes in mice.
Podd BS, Aberg C, Christopher TL, Perez-Cano F, Camerini V. Podd BS, et al. Autoimmunity. 2004 Dec;37(8):537-47. doi: 10.1080/08916930400027094. Autoimmunity. 2004. PMID: 15763916 - MHC class I allele dosage alters CD8 expression by intestinal intraepithelial lymphocytes.
Podd BS, Aberg C, Kudla KL, Keene L, Tobias E, Camerini V. Podd BS, et al. J Immunol. 2001 Sep 1;167(5):2561-8. doi: 10.4049/jimmunol.167.5.2561. J Immunol. 2001. PMID: 11509596 - The CD8 isoform CD8alphaalpha is not a functional homologue of the TCR co-receptor CD8alphabeta.
Gangadharan D, Cheroutre H. Gangadharan D, et al. Curr Opin Immunol. 2004 Jun;16(3):264-70. doi: 10.1016/j.coi.2004.03.015. Curr Opin Immunol. 2004. PMID: 15134773 Review. - Role of the gut as a primary lymphoid organ.
Peaudecerf L, Rocha B. Peaudecerf L, et al. Immunol Lett. 2011 Oct 30;140(1-2):1-6. doi: 10.1016/j.imlet.2011.05.009. Epub 2011 Jun 16. Immunol Lett. 2011. PMID: 21704078 Review.
Cited by
- Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity.
van Wijk F, Cheroutre H. van Wijk F, et al. Semin Immunol. 2009 Jun;21(3):130-8. doi: 10.1016/j.smim.2009.03.003. Epub 2009 Apr 21. Semin Immunol. 2009. PMID: 19386513 Free PMC article. Review. - Destined for the intestine: thymic selection of TCRαβ CD8αα intestinal intraepithelial lymphocytes.
Joannou K, Baldwin TA. Joannou K, et al. Clin Exp Immunol. 2023 Jul 5;213(1):67-75. doi: 10.1093/cei/uxad049. Clin Exp Immunol. 2023. PMID: 37137518 Free PMC article. Review. - Optimal T-cell receptor affinity for inducing autoimmunity.
Koehli S, Naeher D, Galati-Fournier V, Zehn D, Palmer E. Koehli S, et al. Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17248-53. doi: 10.1073/pnas.1402724111. Epub 2014 Nov 19. Proc Natl Acad Sci U S A. 2014. PMID: 25411315 Free PMC article. - Increased TCR signal strength in DN thymocytes promotes development of gut TCRαβ(+)CD8αα(+) intraepithelial lymphocytes.
Grandjean CL, Sumaria N, Martin S, Pennington DJ. Grandjean CL, et al. Sci Rep. 2017 Sep 6;7(1):10659. doi: 10.1038/s41598-017-09368-x. Sci Rep. 2017. PMID: 28878277 Free PMC article. - Positive selection of an MHC class-I restricted TCR in the absence of classical MHC class I molecules.
Maurice MM, Gould DS, Carroll J, Vugmeyster Y, Ploegh HL. Maurice MM, et al. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7437-42. doi: 10.1073/pnas.141143298. Epub 2001 Jun 12. Proc Natl Acad Sci U S A. 2001. PMID: 11404484 Free PMC article.
References
- Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. - PubMed
- Poussier P, Julius M. T-cell development and selection in the intestinal epithelium. Semin Immunol. 1995;7:321–334. - PubMed
- Rocha B, Guy-Grand D, Vassalli P. Extrathymic T cell differentiation. Curr Opin Immunol. 1995;7:235–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous