Heat shock protein 27: developmental regulation and expression after peripheral nerve injury - PubMed (original) (raw)
Heat shock protein 27: developmental regulation and expression after peripheral nerve injury
M Costigan et al. J Neurosci. 1998.
Abstract
The heat shock protein (HSP) 27 is constitutively expressed at low levels in medium-sized lumbar dorsal root ganglion (DRG) cells in adult rats. Transection of the sciatic nerve results in a ninefold upregulation of HSP27 mRNA and protein in axotomized neurons in the ipsilateral DRG at 48 hr, without equivalent changes in the mRNAs encoding HSP56, HSP60, HSP70, and HSP90. Dorsal rhizotomy, injuring the central axon of the DRG neuron, does not upregulate HSP27 mRNA levels. After peripheral axotomy, HSP27 mRNA and protein are present in small, medium, and large DRG neurons, and HSP27 protein is transported anterogradely, accumulating in the dorsal horn and dorsal columns of the spinal cord, where it persists for several months. Axotomized motor neurons also upregulate HSP27. Only a minority of cultured adult DRG neurons are HSP27-immunoreactive soon after dissociation, but all express HSP27 after 24 hr in culture with prominent label throughout the neuron, including the growth cone. HSP27 differs from most axonal injury-regulated and growth-associated genes, which are typically present at high levels in early development and downregulated on innervation of their targets, in that its mRNA is first detectable in the DRG late in development and only approaches adult levels by postnatal day 21. In non-neuronal cells, HSP27 has been shown to be involved both in actin filament dynamics and in protection against necrotic and apoptotic cell death. Therefore, its upregulation after adult peripheral nerve injury may both promote survival of the injured neurons and contribute to alterations in the cytoskeleton associated with axonal growth.
Figures
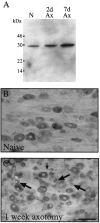
Fig. 1.
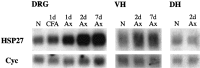
Northern blots for HSP27 and cyclophilin mRNA in L4 and L5 dorsal root ganglia (DRG), ventral horn (VH), and dorsal horn (DH). The DRG lanes contain mRNA (n = 4 animals) from naive unoperated animals (N), animals 1 d after CFA injection (1d CFA), and animals 1, 2, and 7 d after sciatic axotomy (Ax). VH lanes contain mRNA from L4 and L5 ventral horn segments of the spinal cord (n = 3 animals) from naive unoperated animals (N) and animals 2 and 7 d after sciatic cut axotomy (Ax). DH lanes contain mRNA from L4 and L5 dorsal horn segments of the spinal cord (n = 4 animals) from naive unoperated animals (N) and animals 2 d after sciatic cut axotomy (2d Ax). Note the absence of HSP27 mRNA upregulation within the DH 2 d after sciatic nerve axotomy. All experimental material was taken from the same side as the lesion.
Fig. 2.
Northern blots for HSP56, HSP60, HSP70, HSP90, and cyclophilin mRNA in the L4 and L5 dorsal root ganglia (n = 4 animals). Lanes contain mRNA from naive unoperated animals (N) and animals 2 d after sciatic axotomy (2d Ax). Note that in contrast to HSP27 regulation, none of these HSP mRNA are upregulated within the DRG 2 d after sciatic nerve axotomy. HSP56, HSP60, HSP70, and HSP90 mRNA were also not regulated 1 and 7 d after sciatic cut or 1 d after CFA injection in the DRG or in the dorsal horn.
Fig. 3.
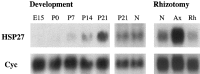
Northern blots for HSP27 and cyclophilin mRNA in L4 and L5 dorsal root ganglia. Development, At E15, no expression is detected. From P0 to P21, HSP27 mRNA gradually increases to levels approximately equal to those seen in the adult (N). Note that the Northern blot for the embryonic and neonatal RNA (E15 to P21) has been exposed for several weeks compared with several days for those of adult RNA (P21 and N).Rhizotomy, Lanes contain mRNA from naive unoperated animals (N), animals 2 d after peripheral axotomy (Ax), and animals 2 d after dorsal root section (Rh). Note the absence of regulation of HSP27 after dorsal root section in contrast to that seen after axotomy of the peripheral nerve. The slight decrease of HSP27 levels in the Rh lane relative to the N lane is attributable to loading differences on the original Northern blot (see Cyc).
Fig. 4.
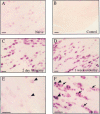
Photomicrographs of 30 μm sections showing HSP27 mRNA expression in the L4 DRG of naive animals (A). Note faint staining in cells across the DRG, many of which are medium and large in size. B, A sense strand HSP27-probed DRG section. C, Forty-eight hours after sciatic nerve section, intensity of HSP27 mRNA staining is dramatically increased, as is the number of cells stained.D, One week after axotomy, staining is still increased. High-power photomicrographs of HSP27 staining in naive ganglia (E) and 48 hr after axotomy (F) show clearly the increase in the intensity of stain. Along with the medium- and large-sized DRG neurons that constitutively express HSP27 (arrowheads), axotomy induces de novo expression in small DRG neurons (arrows). Scale bars, 100 μm.
Fig. 5.
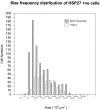
A size frequency histogram of HSP27 mRNA expression in the naive animal and 48 hr after sciatic nerve section. The size frequency histogram shifts to the left after axotomy, reflecting a novel expression of message in smaller cells within the DRG, but there is also a relative increase in large neurons, as well.
Fig. 6.
A, Western blot analysis of DRG for HSP27 protein levels. Lanes contain 20 μg of L4 and L5 DRG protein pooled from naive unoperated animals (N) and animals 2 and 7 d after sciatic axotomy (Ax). Note that the anti-HSP27 antibody recognizes a single band of ∼28 kDa. Positions of molecular weight markers are indicated.B, C, Photomicrographs of 50 μm sections showing HSP27 immunoreactivity in the L4 DRG. In naive animals (B), staining is observed in some DRG neurons, most of which are medium and large in size. Seven days after sciatic nerve section (C), more cells are stained, and consistent with changes seen at the mRNA level, many small cells are positive for HSP27 after axotomy (arrows). Note that HSP27 staining is also observed in the stem axons leaving DRG cell bodies (small arrow). Scale bar, 100 μm.
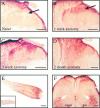
Fig. 7.
Photomicrographs of 50 μm sections showing HSP27 immunoreactivity in the dorsal horn of the L5 segment of spinal cord. In the naive animal (A), fibers are observed in laminae I, III, and IV, in which A-fiber central axon collaterals are known to terminate. Staining is also observed in the dorsal columns. Lamina II is devoid of staining (arrow).B, One week after sciatic nerve section, there is increased HSP27 staining predominantly in the superficial laminae (I and II) of the dorsal horn (arrow). C, Two weeks after axotomy, increased staining is observed in laminae III and IV, as well as in the superficial laminae. Note that the dorsal columns are also densely stained. D, Two months later, HSP27 immunostaining remains in all laminae of the dorsal horn. The dorsal columns ipsilaterally remain heavily stained (compared with contralateral). Scale bars (in A–D), 200 μm.E, Photomicrograph of 50 μm section of sciatic nerve proximal to a ligature 1 week previously. Note that HSP27 has accumulated proximal to the ligature, showing that the protein is anterogradely transported down axons to the periphery.Inset, HSP27 staining in the normal nerve. Scale bar, 1mm. F, HSP27 staining in a spinal cord section 1 week after sciatic nerve section. Along with increased staining in the superficial laminae of the dorsal horn, HSP27 is also upregulated in motor neuron cell bodies of the ventral horn (arrows). Scale bar, 200 μm.
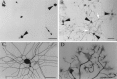
Fig. 8.
HSP27 immunoreactivity in dissociated adult primary DRG cultures. A, One hour after plating the cells. Despite some cells being intensely positive (arrowheads), other neurons are almost completely negative (arrow). B, After 24 hr, all dissociated neurons are positively stained for HSP27 (arrowheads). Note that staining is observed down the entire length of the neurites (white arrows and_C_). Scale bars (in A–C), 100 μm.D, HSP27 staining was also observed in growth cones. Scale bar, 30 μm.
Similar articles
- A role for HSP27 in sensory neuron survival.
Lewis SE, Mannion RJ, White FA, Coggeshall RE, Beggs S, Costigan M, Martin JL, Dillmann WH, Woolf CJ. Lewis SE, et al. J Neurosci. 1999 Oct 15;19(20):8945-53. doi: 10.1523/JNEUROSCI.19-20-08945.1999. J Neurosci. 1999. PMID: 10516313 Free PMC article. - Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo.
Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Dib-Hajj SD, et al. J Neurophysiol. 1998 May;79(5):2668-76. doi: 10.1152/jn.1998.79.5.2668. J Neurophysiol. 1998. PMID: 9582237 - c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy.
Broude E, McAtee M, Kelley MS, Bregman BS. Broude E, et al. Exp Neurol. 1997 Nov;148(1):367-77. doi: 10.1006/exnr.1997.6665. Exp Neurol. 1997. PMID: 9398479 - Expression of auxiliary beta subunits of sodium channels in primary afferent neurons and the effect of nerve injury.
Takahashi N, Kikuchi S, Dai Y, Kobayashi K, Fukuoka T, Noguchi K. Takahashi N, et al. Neuroscience. 2003;121(2):441-50. doi: 10.1016/s0306-4522(03)00432-9. Neuroscience. 2003. PMID: 14522002 - Regulation of expression of galanin and galanin receptors in dorsal root ganglia and spinal cord after axotomy and inflammation.
Zhang X, Xu ZO, Shi TJ, Landry M, Holmberg K, Ju G, Tong YG, Bao L, Cheng XP, Wiesenfeld-Hallin Z, Lozano A, Dostrovsky J, Hökfelt T. Zhang X, et al. Ann N Y Acad Sci. 1998 Dec 21;863:402-13. doi: 10.1111/j.1749-6632.1998.tb10710.x. Ann N Y Acad Sci. 1998. PMID: 9928186 Review.
Cited by
- Neurobiology of glaucomatous optic neuropathy: diverse cellular events in neurodegeneration and neuroprotection.
Wax MB, Tezel G. Wax MB, et al. Mol Neurobiol. 2002 Aug;26(1):45-55. doi: 10.1385/MN:26:1:045. Mol Neurobiol. 2002. PMID: 12392055 Review. - Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth.
Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Tanabe K, et al. J Neurosci. 2003 Oct 22;23(29):9675-86. doi: 10.1523/JNEUROSCI.23-29-09675.2003. J Neurosci. 2003. PMID: 14573547 Free PMC article. - Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons.
Willis D, Li KW, Zheng JQ, Chang JH, Smit AB, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Willis D, et al. J Neurosci. 2005 Jan 26;25(4):778-91. doi: 10.1523/JNEUROSCI.4235-04.2005. J Neurosci. 2005. PMID: 15673657 Free PMC article. - Mode of action of S-methyl-N, N-diethylthiocarbamate sulfoxide (DETC-MeSO) as a novel therapy for stroke in a rat model.
Mohammad-Gharibani P, Modi J, Menzie J, Genova R, Ma Z, Tao R, Prentice H, Wu JY. Mohammad-Gharibani P, et al. Mol Neurobiol. 2014 Oct;50(2):655-72. doi: 10.1007/s12035-014-8658-0. Epub 2014 Feb 28. Mol Neurobiol. 2014. PMID: 24573692 - Effects of ischemic preconditioning on myocardium Caspase-3, SOCS-1, SOCS-3, TNF-α and IL-6 mRNA expression levels in myocardium IR rats.
Ma J, Qiao Z, Xu B. Ma J, et al. Mol Biol Rep. 2013 Oct;40(10):5741-8. doi: 10.1007/s11033-013-2677-1. Epub 2013 Oct 5. Mol Biol Rep. 2013. PMID: 24091940
References
- Aldskogius H, Arvidsson J, Grant G. The reactions of primary sensory neurons to peripheral nerve injury. Brain Res. 1985;373:15–21. - PubMed
- Barr FG, Emanuel BS. Application of a subtraction hybridization technique involving phoactivatable biotin and organic extraction to solution hybridization analysis of genomic DNA. Anal Biochem. 1990;186:369–373. - PubMed
- Benndorf R, Hayes K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein hsp25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. - PubMed
- Brady G, Barbara M, Iscove NN. Representative in vitro cDNA amplification from individual hemopoietic cells and colonies. Methods Mol Cell Biol. 1990;2:17–25.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous