Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice - PubMed (original) (raw)
Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice
E Elstner et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Induction of differentiation and apoptosis in cancer cells through ligands of nuclear hormone receptors (NHRs) is a novel and promising approach to cancer therapy. All-trans-retinoic acid (ATRA), an RA receptor-specific NHR ligand, is now used for selective cancers. The NHR, peroxisome proliferator-activated receptor gamma (PPARgamma) is expressed in breast cancer cells. Activation of PPARgamma through a synthetic ligand, troglitazone (TGZ), and other PPARgamma-activators cause inhibition of proliferation and lipid accumulation in cultured breast cancer cells. TGZ (10(-5) M, 4 days) reversibly inhibits clonal growth of MCF7 breast cancer cells and the combination of TGZ (10(-5) M) and ATRA (10(-6) M, 4 days) synergistically and irreversibly inhibits growth and induces apoptosis of MCF7 cells, associated with a dramatic decrease of their bcl-2 protein levels. Similar effects are noted with in vitro cultured breast cancer tissues from patients, but not with normal breast epithelial cells. The observed apoptosis mediated by TGZ and ATRA may be related to the striking down-regulation of bcl-2, because forced over-expression of bcl-2 in MCF7 cells cultured with TGZ and ATRA blocks their cell death. TGZ significantly inhibits MCF7 tumor growth in triple immunodeficient mice. Combined administration of TGZ and ATRA causes prominent apoptosis and fibrosis of these tumors without toxic effects on the mice. Taken together, this combination may provide a novel, nontoxic and selective therapy for human breast cancers.
Figures
Figure 1
Expression of PPARγ protein in infiltrating ductal breast adenocarcinoma. Benign breast ducts show low immunoreactivity (arrow 1), whereas infiltrating carcinoma cells are strongly positive (arrow 2).
Figure 2
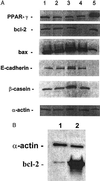
Expression of PPARγ protein in human breast cancer cell lines. Lane 1, human breast adipocytes (positive control); lane 2, T47D cells; lane 3, MCF7 cells; lane 4, MDA-MB-231 cells; lane 5, BT474 cells. The α-actin is control for the amount of loaded protein.
Figure 3
Dose-response effect of TGZ and/or ATRA on clonal proliferation of breast cancer cell lines Results are expressed as the mean percentage of colonies in control plates containing no ligand. Each point represents mean ± SD of three independent experiments with triplicate dishes.
Figure 4
Pulse-exposure of MCF7 cells to TGZ (10−5 M), indomethacin (IND) (10−5 M), 15d-PGJ2 (10−5 M), and/or ATRA(10−6 M). Results are expressed as the mean percentage of control plates containing no ligand. Each point represents a mean ± SD of three independent experiments with triplicate dishes.
Figure 5
(A) Expression of PPARγ, bcl-2, bax, E-cadherin, and β-casein in MCF7 cells after their incubation with ligands for 4 days as measured by Western blot. Lane 1, control (vehicle alone); lane 2, TGZ (10−5 M); lane 3, ATRA (10−6 M); lane 4, TGZ (10−5 M) +ATRA (10−6 M); lane 5, human breast adipocytes (positive control). (B) Expression of bcl-2 protein in bcl-2 transfected MCF7 cells as measured by Western blot (20 μg of protein per lane).
Figure 6
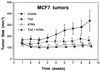
Effect of TGZ and ATRA, either alone or in combination on the size of MCF7 tumors in BNX triple immunodeficient mice. Results represent the mean ± SD of 10 tumors.
Figure 7
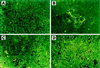
Apoptotic cells in MCF7 tumors in BNX triple immunodeficient mice as measured by TUNEL assay. control mice (A); treated with TGZ (B); ATRA (C); combination of both ligands (D).
Similar articles
- Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo.
Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP. Kubota T, et al. Cancer Res. 1998 Aug 1;58(15):3344-52. Cancer Res. 1998. PMID: 9699665 - Thiazolidinediones inhibit growth of gastrointestinal, biliary, and pancreatic adenocarcinoma cells through activation of the peroxisome proliferator-activated receptor gamma/retinoid X receptor alpha pathway.
Tsujie M, Nakamori S, Okami J, Hayashi N, Hiraoka N, Nagano H, Dono K, Umeshita K, Sakon M, Monden M. Tsujie M, et al. Exp Cell Res. 2003 Sep 10;289(1):143-51. doi: 10.1016/s0014-4827(03)00263-5. Exp Cell Res. 2003. PMID: 12941612 - Magnitude of peroxisome proliferator-activated receptor-gamma activation is associated with important and seemingly opposite biological responses in breast cancer cells.
Clay CE, Namen AM, Atsumi G, Trimboli AJ, Fonteh AN, High KP, Chilton FH. Clay CE, et al. J Investig Med. 2001 Sep;49(5):413-20. doi: 10.2310/6650.2001.33786. J Investig Med. 2001. PMID: 11523697 - Combined treatment with specific ligands for PPARgamma:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells.
Mu YM, Yanase T, Nishi Y, Takayanagi R, Goto K, Nawata H. Mu YM, et al. Mol Cell Endocrinol. 2001 Jul 5;181(1-2):239-48. doi: 10.1016/s0303-7207(00)00457-3. Mol Cell Endocrinol. 2001. PMID: 11476957
Cited by
- PPAR Could Contribute to the Pathogenesis of Hepatocellular Carcinoma.
Kimura O, Kondo Y, Shimosegawa T. Kimura O, et al. PPAR Res. 2012;2012:574180. doi: 10.1155/2012/574180. Epub 2012 Dec 16. PPAR Res. 2012. PMID: 23316217 Free PMC article. - Solid tumor differentiation therapy - is it possible?
Cruz FD, Matushansky I. Cruz FD, et al. Oncotarget. 2012 May;3(5):559-67. doi: 10.18632/oncotarget.512. Oncotarget. 2012. PMID: 22643847 Free PMC article. Review. - Clinical relevance of peroxisome proliferator-activated receptor-gamma expression in myxoid liposarcoma.
Takeuchi A, Yamamoto N, Shirai T, Hayashi K, Miwa S, Munesue S, Yamamoto Y, Tsuchiya H. Takeuchi A, et al. BMC Cancer. 2016 Jul 11;16:442. doi: 10.1186/s12885-016-2524-6. BMC Cancer. 2016. PMID: 27401457 Free PMC article. - Immunohistochemical analysis of thymic carcinoma focusing on the possibility of molecular targeted and hormonal therapies.
Omatsu M, Kunimura T, Mikogami T, Hamatani S, Shiokawa A, Masunaga A, Kitami A, Suzuki T, Kadokura M, Morohoshi T. Omatsu M, et al. Gen Thorac Cardiovasc Surg. 2012 Dec;60(12):803-10. doi: 10.1007/s11748-012-0160-x. Epub 2012 Oct 3. Gen Thorac Cardiovasc Surg. 2012. PMID: 23054618 - MAZ drives tumor-specific expression of PPAR gamma 1 in breast cancer cells.
Wang X, Southard RC, Allred CD, Talbert DR, Wilson ME, Kilgore MW. Wang X, et al. Breast Cancer Res Treat. 2008 Sep;111(1):103-11. doi: 10.1007/s10549-007-9765-7. Epub 2007 Sep 28. Breast Cancer Res Treat. 2008. PMID: 17902047 Free PMC article.
References
- Warrell R P, Jr, Frankel S R, Miller W H, Jr, Scheinberg D A, Itri L M, Hittelman W N, Vyas R, Andreeff M, Tafuri A, Jakubowski A, et al. N Engl J Med. 1991;324:1385–1393. - PubMed
- Hong W K, Lippman S M, Itri L M, Karp D D, Lee J S, Byers R M, Schantz S P, Kramer A M, Lotan R, Peters L J, et al. N Engl J Med. 1990;323:795–801. - PubMed
- Lippman S M, Kavanagh J J, Paredes-Espinoza M, Delgadillo-Madrueno F, Paredes-Casillas P, Hong W K, Holdener E, Krakoff I. J Natl Cancer Inst. 1992;84:241–245. - PubMed
- Lippman S M, Parkinson D R, Itri L M, Weber R S, Schantz S P, Ota D M, Schusterman M A, Krakoff I H, Gutterman J U, Hong W K J. J Natl Cancer Inst. 1992;84:235–240. - PubMed
- Heyman R A, Mangelsdorf D J, Dyck J A, Stein R B, Eichele G, Evans R M, Thaller C. Cell. 1992;68:397–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical