Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth - PubMed (original) (raw)
Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth
R J Thompson et al. Infect Immun. 1998 Aug.
Abstract
Listeria monocytogenes is an intracellular bacterial pathogen that elicits a strong cellular immune response following infection and therefore has potential use as a vaccine vector. However, while infections by L. monocytogenes are fairly rare and can readily be controlled by a number of antibiotics, the organism can nevertheless cause meningitis and death, particularly in immunocompromised or pregnant patients. We therefore have endeavored to isolate a highly attenuated strain of this organism for use as a vaccine vector. D-Alanine is required for the synthesis of the mucopeptide component of the cell walls of virtually all bacteria and is found almost exclusively in the microbial world. We have found in L. monocytogenes two genes that control the synthesis of this compound, an alanine racemase gene (dal) and a D-amino acid aminotransferase gene (dat). By inactivating both genes, we produced an organism that could be grown in the laboratory when supplemented with D-alanine but was unable to grow outside the laboratory, particularly in the cytoplasm of eukaryotic host cells, the natural habitat of this organism during infection. In mice, the double-mutant strain was completely attenuated. Nevertheless, it showed the ability, particularly under conditions of transient suppression of the mutant phenotype, to induce cytotoxic T-lymphocyte responses and to generate protective immunity against lethal challenge by wild-type L. monocytogenes equivalent to that induced by the wild-type organism.
Figures
FIG. 1
Nucleotide sequence and translation of the alanine racemase (dal) gene of L. monocytogenes. The gene was inactivated either by insertion of a 1.35-kb fragment of DNA encoding erythromycin resistance at a _Spe_I site at nucleotide 517 or by deletion of nucleotides 44 to 948.
FIG. 2
Linear alignment of deduced protein sequences of alanine racemases of L. monocytogenes (LMDAL), B. stearothermophilus (BSTDAL), and B. subtilis (BSUBDAL). Identical amino acids are boxed.
FIG. 3
Nucleotide sequence and translation of the
d
-amino acid aminotransferase (dat) gene of L. monocytogenes. The gene was inactivated by deletion of nucleotides 370 to 636.
FIG. 4
Linear alignment of deduced protein sequences of
d
-amino acid aminotransferases of L. monocytogenes (LMDAT), S. haemolyticus (SHAEDAT), B. sphaericus (BSPHDAT), and Bacillus sp. strain YM-1 (BSPDAT). Identical amino acids are boxed.
FIG. 5
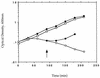
Growth requirement for
d
-alanine of the dal dat double-mutant strain of L. monocytogenes. The dal dat (○, •) and wild-type (□) strains of L. monocytogenes were grown in liquid culture (BHI medium) in the presence (•) or absence (○, □) of exogenous
d
-alanine (100 μg/ml) at 37°C. An aliquot of the mutant culture was provided
d
-alanine at 90 min. The starting cultures were in log phase of growth. As
d
-alanine had no effect on the growth of wild-type L. monocytogenes, those data are not shown.
FIG. 6
Light micrographs showing the growth of wild-type (A) and dal dat double-mutant (B) strains of L. monocytogenes in J774 macrophages at 5 h after infection (at approximately 5 bacteria per mouse cell). (C) Infection by double-mutant bacteria in the continuous presence of
d
-alanine (80 μg/ml).
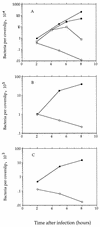
FIG. 7
Infection of mammalian cells with the dal dat double-mutant (○) and wild-type strains of L. monocytogenes (•). (A) J774 murine macrophage-like cells (MOI of about 0.05). Mutant infection in one culture (■) was in the continuous presence of
d
-alanine (100 μg/ml); cells in an aliquot of that culture (□) were resuspended in
d
-alanine-free medium at 4 h. (B) Primary murine bone marrow-derived macrophages (MOI of about 5). (C) Human epithelial cells (HeLa) (MOI of about 5). Starting cultures of L. monocytogenes were in stationary phase of growth.
FIG. 8
Association of actin with intracytosolic wild-type L. monocytogenes (A, 2 h; B, 5 h) or with the dal dat double mutant (C, 2 h with
d
-alanine [100 μg/ml] present from 0 to 30 min; D, 5 h with
d
-alanine present from 0 to 30 min; E, 5 h with
d
-alanine present continuously) following infection of J774 cells. Photomicrographs in the top row show the binding of FITC-labeled antilisterial antibodies to total bacteria; those below show the binding of TRITC-labeled phalloidin to actin. Arrowheads indicate some actin-associated bacteria in the sparsely infected cells.
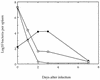
FIG. 9
Recovery of bacteria from spleens of BALB/c mice following sublethal infection with wild-type L. monocytogenes (•), the dal dat mutant in the absence of
d
-alanine (○), and the dal dat mutant in the presence of 20 mg of
d
-alanine in the inoculation fluid (□). The points at day 0 show the total number of viable organisms injected, not bacteria per spleen.
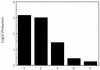
FIG. 10
Protection of BALB/c mice against challenge with 10 × LD50 of wild-type L. monocytogenes by immunization with the dal dat double-mutant strain of L. monocytogenes. Column numbers represent groups of five mice immunized with the following organisms: 1, 4 × 102 CFU of wild-type L. monocytogenes; 2, 2 × 107 CFU of dal dat mutant (plus 20 mg of
d
-ala), 3, 2 × 105 CFU of dal dat mutant (plus
d
-ala); 4, 2 × 104 CFU of dal dat mutant (plus
d
-ala), 5, 2 × 107 CFU of dal dat mutant (no
d
-ala). Mice were challenged 21 to 28 days later. Log10 protection was calculated as described in Materials and Methods. The largest error seen in all mouse groups was 0.17 log10.
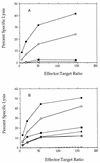
FIG. 11
Cytolytic activity of splenocytes isolated from mice 10 to 14 days after infection with wild-type L. monocytogenes (•, ○) or naive controls (■, □) (A). Open and closed symbols represent independent experiments;
d
-alanine was not provided in either experiment. (B) dal dat double mutant: 3 × 107 bacteria (+), 3 × 107 bacteria with boost at 10 days (▴, ▵); 3 × 107 bacteria with animals provided
d
-alanine subcutaneously (40 mg at −6 h, time of infection, 6 h, and 12 h) (•, ○), 3 × 107 bacteria with
d
-alanine (2 mg/ml; ■) or
d
-alanine (0.2 mg/ml; □) in drinking water from 24 h before infection to 36 h post-infection. Open and closed symbols represent independent experiments.
Similar articles
- Pathogenicity and immunogenicity of a vaccine strain of Listeria monocytogenes that relies on a suicide plasmid to supply an essential gene product.
Zhao X, Li Z, Gu B, Frankel FR. Zhao X, et al. Infect Immun. 2005 Sep;73(9):5789-98. doi: 10.1128/IAI.73.9.5789-5798.2005. Infect Immun. 2005. PMID: 16113297 Free PMC article. - Conditional lethality yields a new vaccine strain of Listeria monocytogenes for the induction of cell-mediated immunity.
Li Z, Zhao X, Higgins DE, Frankel FR. Li Z, et al. Infect Immun. 2005 Aug;73(8):5065-73. doi: 10.1128/IAI.73.8.5065-5073.2005. Infect Immun. 2005. PMID: 16041022 Free PMC article. - A truncated Bacillus subtilis dal gene with a 3' ssrA gene tag regulates the growth and virulence of racemase-deficient Listeria monocytogenes.
Li Z, Zhao X, Zhou C, Gu B, Frankel FR. Li Z, et al. Microbiology (Reading). 2006 Oct;152(Pt 10):3091-3102. doi: 10.1099/mic.0.28994-0. Microbiology (Reading). 2006. PMID: 17005988 - Induction of human immunodeficiency virus (HIV)-specific CD8 T-cell responses by Listeria monocytogenes and a hyperattenuated Listeria strain engineered to express HIV antigens.
Friedman RS, Frankel FR, Xu Z, Lieberman J. Friedman RS, et al. J Virol. 2000 Nov;74(21):9987-93. doi: 10.1128/jvi.74.21.9987-9993.2000. J Virol. 2000. PMID: 11024127 Free PMC article. - Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells?
Chávez-Arroyo A, Portnoy DA. Chávez-Arroyo A, et al. Cell Microbiol. 2020 Apr;22(4):e13175. doi: 10.1111/cmi.13175. Cell Microbiol. 2020. PMID: 32185899 Free PMC article. Review.
Cited by
- (D)-Amino acid chemical reporters reveal peptidoglycan dynamics of an intracellular pathogen.
Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR. Siegrist MS, et al. ACS Chem Biol. 2013 Mar 15;8(3):500-5. doi: 10.1021/cb3004995. Epub 2013 Jan 11. ACS Chem Biol. 2013. PMID: 23240806 Free PMC article. - Multi-virulence-locus sequence typing of Listeria monocytogenes.
Zhang W, Jayarao BM, Knabel SJ. Zhang W, et al. Appl Environ Microbiol. 2004 Feb;70(2):913-20. doi: 10.1128/AEM.70.2.913-920.2004. Appl Environ Microbiol. 2004. PMID: 14766571 Free PMC article. - Enhancing mucosal immunity by transient microbiota depletion.
Becattini S, Littmann ER, Seok R, Amoretti L, Fontana E, Wright R, Gjonbalaj M, Leiner IM, Plitas G, Hohl TM, Pamer EG. Becattini S, et al. Nat Commun. 2020 Sep 8;11(1):4475. doi: 10.1038/s41467-020-18248-4. Nat Commun. 2020. PMID: 32901029 Free PMC article. - A Cross-Protective Vaccine Against 4b and 1/2b Listeria monocytogenes.
Meng F, Zhu T, Yao H, Ling Z, Feng Y, Li G, Li J, Sun X, Chen J, Meng C, Jiao X, Yin Y. Meng F, et al. Front Microbiol. 2020 Dec 11;11:569544. doi: 10.3389/fmicb.2020.569544. eCollection 2020. Front Microbiol. 2020. PMID: 33362730 Free PMC article. - Pathogenicity and immunogenicity of a vaccine strain of Listeria monocytogenes that relies on a suicide plasmid to supply an essential gene product.
Zhao X, Li Z, Gu B, Frankel FR. Zhao X, et al. Infect Immun. 2005 Sep;73(9):5789-98. doi: 10.1128/IAI.73.9.5789-5798.2005. Infect Immun. 2005. PMID: 16113297 Free PMC article.
References
- Ada G L. The immunological principles of vaccination. Lancet. 1990;335:523–526. - PubMed
- Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. - PubMed
- Braciale T J, Morrison L A, Sweetser M T, Sambrook J, Gething M J, Braciale V L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987;98:95–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources