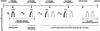Chromosome methylation patterns during mammalian preimplantation development - PubMed (original) (raw)
Chromosome methylation patterns during mammalian preimplantation development
N Rougier et al. Genes Dev. 1998.
Abstract
DNA methylation patterns were evaluated during preimplantation mouse development by analyzing the binding of monoclonal antibody to 5-methylcytosine (5-MeC) on metaphase chromosomes. Specific chromosome patterns were observed in each cell stage. A banding pattern predominated in chromosomes at the one-cell stage. Banding was replaced at the two-cell stage by an asymmetrical labeling of the sister chromatids. Then, the proportion of asymmetrical chromosomes decreased by one-half at each cell division until the blastocyst stage, and chromosomes became progressively symmetrical and weakly labeled. Our results indicate that chromosome demethylation is associated with each DNA replication and suggest that a passive mechanism predominates during early development.
Figures
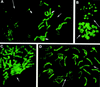
Figure 1
Chromosome methylation patterns of mouse embryos at the one-cell stage. Methylated sites were revealed by indirect immunofluorescence labeling with 5-MeC monoclonal antibody. (A,B) Metaphases from normal mouse embryos obtained without (A) and with (B) colchicine treatment. (A) Two distinct sets of 20 chromosomes each are observed (in mouse 2n = 40). One chromosome set displays an intense R-like banding pattern (right, medium thin arrow). The other set is faintly labeled (left, long thin arrow). Centromeric heterochromatin (small thick arrows) is labeled intensely in only a few chromosomes of the first set, whereas it is labeled in most chromosomes of the second set. (C) Parthenogenetic embryos: all chromosomes display an R-like banding pattern confirming that more labeled chromosomes in normal embryos are of maternal origin. (D) Chromosomes from an embryo carrying Robertsonian translocations of paternal origin: Normal maternal chromosomes (medium thin arrow) are labeled more intensely than paternal translocated chromosomes (long thin arrow).
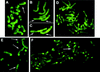
Figure 2
Chromosome methylation patterns of mouse embryos at the two-, four- and eight-cell stages. 5-MeC antibody binding and indirect immunofluorescence detection. (A–D) Chromosomes from two-cell embryos showing an asymmetrical labeling of the two sister chromatids (arrows). In some chromosomes labeling similar to sister chromatid exchanges can be seen. (A) Chromosomes of maternal origin from a normal mouse embryo. (B,C) Chromosomes from embryos carrying Robertsonian translocations of paternal origin. Paternal chromosomes are faintly labeled in relation to maternal chromosomes. In C fluorescence of the same paternal chromosomes showed in B was intensified. (D) Metaphase from a parthenogenetic embryo showing the asymmetrical labeling of maternal chromosomes. (E) Chromosomes from a four-cell embryo: Asymmetrical (arrows) and faintly labeled chromosomes are observed. (F) Metaphase from a eight-cell embryo: Asymmetrical chromosomes are rare (arrow).
Figure 3
Diagram of the chromosome methylation changes occurring during preimplantation development of mouse embryos. (A) Chromosome patterns obtained after 5-MeC antibody binding. (B) Summary of DNA methylation alterations that are compatible with the observed chromosome patterns. (A) At the one-cell stage, maternal and paternal chromosomes display heterogeneous but symmetrical labeling of chromatids. Maternal chromosomes are labeled more intensely than paternal chromosomes and display an R-like banding pattern. At the two-cell stage, maternal and paternal chromosomes become asymmetrically labeled, maternal chromosomes always being stained more strongly than paternal chromosomes. From the four-cell stage to blastocyst the number of asymmetrical chromosomes halves after each S phase. At morula (16/32 cells) and blastocyst stages, two cell populations differing by their chromosome condensation can be distinguished: in the first, chromosomes are normally condensed (A, left); in the second, chromosomes are thick and their condensation seems relaxed (A, right). (B) The presence of asymmetrical chromosomes after two S-phases following fertilization (two-cell stage) indicates that after the first S phase (one-cell stage, first metaphase), DNA is hemimethylated in both chromatids, and after the second S phase (two-cell stage, second metaphase), DNA is hemimethylated in one chromatid and demethylated in the other one. As the number of asymmetrical chromosomes halve as cleavage progresses, a failure in maintenance activity on the new DNA strand of each S phase can explain the progression of demethylation. (S) Sphase; (○) unmethylated CpGs; (•) methylated CpGs.
Figure 3
Diagram of the chromosome methylation changes occurring during preimplantation development of mouse embryos. (A) Chromosome patterns obtained after 5-MeC antibody binding. (B) Summary of DNA methylation alterations that are compatible with the observed chromosome patterns. (A) At the one-cell stage, maternal and paternal chromosomes display heterogeneous but symmetrical labeling of chromatids. Maternal chromosomes are labeled more intensely than paternal chromosomes and display an R-like banding pattern. At the two-cell stage, maternal and paternal chromosomes become asymmetrically labeled, maternal chromosomes always being stained more strongly than paternal chromosomes. From the four-cell stage to blastocyst the number of asymmetrical chromosomes halves after each S phase. At morula (16/32 cells) and blastocyst stages, two cell populations differing by their chromosome condensation can be distinguished: in the first, chromosomes are normally condensed (A, left); in the second, chromosomes are thick and their condensation seems relaxed (A, right). (B) The presence of asymmetrical chromosomes after two S-phases following fertilization (two-cell stage) indicates that after the first S phase (one-cell stage, first metaphase), DNA is hemimethylated in both chromatids, and after the second S phase (two-cell stage, second metaphase), DNA is hemimethylated in one chromatid and demethylated in the other one. As the number of asymmetrical chromosomes halve as cleavage progresses, a failure in maintenance activity on the new DNA strand of each S phase can explain the progression of demethylation. (S) Sphase; (○) unmethylated CpGs; (•) methylated CpGs.
Similar articles
- [Peculiarities of metaphase chromosome methylation pattern in preimplantation human embryos].
Baranov VS, Pendina AA, Kuznetsova TV, Efimova OA, Fedorova ID, Leont'eva OA, Korsak VS, Nikol'skiĭ NN. Baranov VS, et al. Tsitologiia. 2005;47(8):723-30. Tsitologiia. 2005. PMID: 16706217 Russian. - Distribution of 5-methylcytosine-rich regions in the metaphase chromosomes of Vicia faba.
Frediani M, Giraldi E, Castiglione MR. Frediani M, et al. Chromosome Res. 1996 Feb;4(2):141-6. doi: 10.1007/BF02259707. Chromosome Res. 1996. PMID: 8785608 - DNA methylation patterns of metaphase chromosomes in human preimplantation embryos.
Pendina AA, Efimova OA, Fedorova ID, Leont'eva OA, Shilnikova EM, Lezhnina JG, Kuznetzova TV, Baranov VS. Pendina AA, et al. Cytogenet Genome Res. 2011;132(1-2):1-7. doi: 10.1159/000318673. Epub 2010 Aug 17. Cytogenet Genome Res. 2011. PMID: 20720394 - DNA methylation in the preimplantation embryo: the differing stories of the mouse and sheep.
Young LE, Beaujean N. Young LE, et al. Anim Reprod Sci. 2004 Jul;82-83:61-78. doi: 10.1016/j.anireprosci.2004.05.020. Anim Reprod Sci. 2004. PMID: 15271444 Review. - DNA methylation and polyamines in embryonic development and cancer.
Heby O. Heby O. Int J Dev Biol. 1995 Oct;39(5):737-57. Int J Dev Biol. 1995. PMID: 8645558 Review.
Cited by
- Developmental toxicant exposures and sex-specific effects on epigenetic programming and cardiovascular health across generations.
Svoboda LK, Ishikawa T, Dolinoy DC. Svoboda LK, et al. Environ Epigenet. 2022 Oct 3;8(1):dvac017. doi: 10.1093/eep/dvac017. eCollection 2022. Environ Epigenet. 2022. PMID: 36325489 Free PMC article. Review. - Intergenerational association of DNA methylation between parents and offspring.
Jiang Y, Zhang H, Chen S, Ewart S, Holloway JW, Arshad H, Karmaus W. Jiang Y, et al. Sci Rep. 2024 Aug 27;14(1):19812. doi: 10.1038/s41598-024-69317-3. Sci Rep. 2024. PMID: 39191877 Free PMC article. - Inhibition of MEK1/2 and GSK3 (2i system) affects blastocyst quality and early differentiation of porcine parthenotes.
Kwon J, Li YH, Jo YJ, Oh Y, Namgoong S, Kim NH. Kwon J, et al. PeerJ. 2019 Jan 7;6:e5840. doi: 10.7717/peerj.5840. eCollection 2019. PeerJ. 2019. PMID: 30643672 Free PMC article. - Limited demethylation leaves mosaic-type methylation states in cloned bovine pre-implantation embryos.
Kang YK, Park JS, Koo DB, Choi YH, Kim SU, Lee KK, Han YM. Kang YK, et al. EMBO J. 2002 Mar 1;21(5):1092-100. doi: 10.1093/emboj/21.5.1092. EMBO J. 2002. PMID: 11867537 Free PMC article. - Silencing inhibits Cre-mediated recombination of the Z/AP and Z/EG reporters in adult cells.
Long MA, Rossi FM. Long MA, et al. PLoS One. 2009;4(5):e5435. doi: 10.1371/journal.pone.0005435. Epub 2009 May 5. PLoS One. 2009. PMID: 19415111 Free PMC article.
References
- Adenot PG, Mercier Y, Renard JP, Thompson EM. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development. 1997;124:4615–4625. - PubMed
- Beechey CV, Evans EB. Chromosome variants. In: Lyon M, Rastan S, Brown S, editors. Genetic variants and strains of the laboratory mouse. Oxford, UK: Oxford University Press; 1996. p. 1483.
- Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources