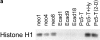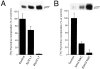E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) - PubMed (original) (raw)
E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1)
B St Croix et al. J Cell Biol. 1998.
Abstract
Recent studies have demonstrated the importance of E-cadherin, a homophilic cell-cell adhesion molecule, in contact inhibition of growth of normal epithelial cells. Many tumor cells also maintain strong intercellular adhesion, and are growth-inhibited by cell- cell contact, especially when grown in three-dimensional culture. To determine if E-cadherin could mediate contact-dependent growth inhibition of nonadherent EMT/6 mouse mammary carcinoma cells that lack E-cadherin, we transfected these cells with an exogenous E-cadherin expression vector. E-cadherin expression in EMT/6 cells resulted in tighter adhesion of multicellular spheroids and a reduced proliferative fraction in three-dimensional culture. In addition to increased cell-cell adhesion, E-cadherin expression also resulted in dephosphorylation of the retinoblastoma protein, an increase in the level of the cyclin-dependent kinase inhibitor p27(kip1) and a late reduction in cyclin D1 protein. Tightly adherent spheroids also showed increased levels of p27 bound to the cyclin E-cdk2 complex, and a reduction in cyclin E-cdk2 activity. Exposure to E-cadherin-neutralizing antibodies in three-dimensional culture simultaneously prevented adhesion and stimulated proliferation of E-cadherin transfectants as well as a panel of human colon, breast, and lung carcinoma cell lines that express functional E-cadherin. To test the importance of p27 in E-cadherin-dependent growth inhibition, we engineered E-cadherin-positive cells to express inducible p27. By forcing expression of p27 levels similar to those observed in aggregated cells, the stimulatory effect of E-cadherin-neutralizing antibodies on proliferation could be inhibited. This study demonstrates that E-cadherin, classically described as an invasion suppressor, is also a major growth suppressor, and its ability to inhibit proliferation involves upregulation of the cyclin-dependent kinase inhibitor p27.
Figures
Figure 1
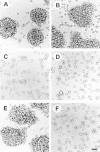
Morphology of EMT/6 cells displaying either E-cadherin or HA-dependent intercellular adhesion in three-dimensional culture. (a–c) EMT/6 cells transfected with E-cadherin (Ecad9); (d) with neomycin vector alone (neo8), or (e and f) Pc5-T cells with HA-dependent adhesion were cultured with 1,000 U/ml hyaluronidase (a–d, f), 10 μg/ml DECMA-1 antibody (c and e), or 10 μg/ml of GoH3 anti-α6-integrin antibody (b). Cells were photographed after 24 h in three-dimensional culture. Bar, 50 μm.
Figure 2
Immunoblot analysis of E-cadherin and β-catenin in EMT/6 cells. EMT/6 neomycin-resistant control transfectants (neo1, neo4), E-cadherin transfectants (Ecad3, Ecad7, or Ecad9), or EMT/6 loosely adherent (Pc10-L and Pc11-L) or tightly adherent (Pc5-T, PcT-7) variants were grown for 48 h in three-dimensional culture. E-cadherin–dependent adhesion was prevented by treatment with DECMA-1 antibody (Dec), and HA-dependent adhesion was prevented with hyaluronidase (Hy). In the top panel, anti-E-cadherin and anti-β-catenin antibodies detected proteins of 120 and 95 kD, respectively, by Western blot analysis. When cell lysates were immunoprecipitated with E-cadherin antibodies and then immunoblotted with anti-E-cadherin and anti-β-catenin antibodies (bottom), again bands of 120 and 95 kD were detected.
Figure 3
E-cadherin suppresses growth of EMT/6 cells in three-dimensional culture. (a) Proliferation of EMT/6 cells transfected with E-cadherin (_Ecad_9) or neomycin vector alone (neo1) as measured by BrdU incorporation into DNA. 24 h after plating in three-dimensional culture, cells were either untreated (−BrdU) or pulsed with BrdU (+BrdU) for a further 24 h. Cells were prepared as described in the Materials and Methods, and cell cycle profiles were analyzed by flow cytometry. Tightly adherent or hyaluronidase (HYase) dispersed Pc5-T cells were also included as an additional control. (b) Proliferation of neomycin-resistant control transfectants (neo4, neo7, neo8) or E-cadherin transfectants (Ecad3, Ecad7, Ecad9, and Ecad18) as measured by [3H]thymidine incorporation into DNA. Intercellular adhesion of E-cadherin transfectants was prevented by treatment with 2 μg/ml DECMA-1 antibody at the time of plating.
Figure 3
E-cadherin suppresses growth of EMT/6 cells in three-dimensional culture. (a) Proliferation of EMT/6 cells transfected with E-cadherin (_Ecad_9) or neomycin vector alone (neo1) as measured by BrdU incorporation into DNA. 24 h after plating in three-dimensional culture, cells were either untreated (−BrdU) or pulsed with BrdU (+BrdU) for a further 24 h. Cells were prepared as described in the Materials and Methods, and cell cycle profiles were analyzed by flow cytometry. Tightly adherent or hyaluronidase (HYase) dispersed Pc5-T cells were also included as an additional control. (b) Proliferation of neomycin-resistant control transfectants (neo4, neo7, neo8) or E-cadherin transfectants (Ecad3, Ecad7, Ecad9, and Ecad18) as measured by [3H]thymidine incorporation into DNA. Intercellular adhesion of E-cadherin transfectants was prevented by treatment with 2 μg/ml DECMA-1 antibody at the time of plating.
Figure 4
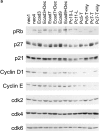
Changes in cell cycle molecules in response to intercellular adhesion of EMT/6 cells as detected by immunoblotting. (a) Three-dimensional cultures of EMT/6 neomycin–resistant control transfectants (neo1, neo4), E-cadherin transfectants (Ecad3, Ecad7, and Ecad9), EMT/6 loosely-adherent variants (Pc10-L and Pc11-L) or HA-dependent tightly adherent variants (Pc5-T, PcT-7) were examined. Immunoblots were probed for the cell cycle molecules indicated. E-cadherin- or HA-dependent intercellular adhesion was prevented by treatment with either DECMA-1 antibody (Dec) or hyaluronidase (Hy), respectively. Note changes in pRb phosphorylation and levels of cyclin D1 and p27 in cells allowed to aggregate in three-dimensional culture. (b) Time course demonstrating changes in p27 and cyclin D1 protein levels as cells aggregate in three-dimensional culture. Cells transfected with E-cadherin (Ecad3) grown in the presence or absence of 2 μg/ml DECMA-1 antibody for the indicated times were probed for p27 and cyclin D1 by immunoblotting. At time = 0 cells were transferred from two- to three-dimensional culture.
Figure 4
Changes in cell cycle molecules in response to intercellular adhesion of EMT/6 cells as detected by immunoblotting. (a) Three-dimensional cultures of EMT/6 neomycin–resistant control transfectants (neo1, neo4), E-cadherin transfectants (Ecad3, Ecad7, and Ecad9), EMT/6 loosely-adherent variants (Pc10-L and Pc11-L) or HA-dependent tightly adherent variants (Pc5-T, PcT-7) were examined. Immunoblots were probed for the cell cycle molecules indicated. E-cadherin- or HA-dependent intercellular adhesion was prevented by treatment with either DECMA-1 antibody (Dec) or hyaluronidase (Hy), respectively. Note changes in pRb phosphorylation and levels of cyclin D1 and p27 in cells allowed to aggregate in three-dimensional culture. (b) Time course demonstrating changes in p27 and cyclin D1 protein levels as cells aggregate in three-dimensional culture. Cells transfected with E-cadherin (Ecad3) grown in the presence or absence of 2 μg/ml DECMA-1 antibody for the indicated times were probed for p27 and cyclin D1 by immunoblotting. At time = 0 cells were transferred from two- to three-dimensional culture.
Figure 5
Increased p27 binding to cyclin E mediates reduced Cyclin E–associated kinase activity in aggregated cells. (a) Cyclin E–associated cdk2 kinase activity, as measured by phosphorylation of Histone H1 substrate in representative neomycin-resistant control transfectants (neo1, neo4, neo8) or E-cadherin transfectants (Ecad1, Ecad9, Ecad18) grown in three-dimensional culture. Also included are Pc5-T cells grown in monolayer (2-D) culture, or in three-dimensional culture in either the presence or absence of hyaluronidase (Hy). (b) Increased levels of p27 bound to cyclin E in tightly adherent EMT/6 spheroids. After immunoprecipitating cyclin E from tightly- or loosely adherent aggregates, levels of cyclin E–bound p27 were detected by immunoblotting. Cell-free lysis buffer was used for the control sample. (c) Immunodepletion of p27 from aggregated E-cadherin transfectants. Cell extracts immunodepleted one (1×) or two (2×) times with antibodies against p27 were probed for p27 by immunoblotting. Controls (Con) represent extracts before immunodepletion (d). Cyclin E, cyclin E–associated cdk2, and p27 are markedly reduced in p27-immunodepleted (ID) supernatents from E-cadherin–aggregated cells. Cyclin E immunoprecipitates from lysates with or without prior immunodepletion with p27 antibodies were probed for cyclin E, cdk2, or p27 by immunoblotting. As a control, Ecad9 lysates were immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. (e) Cyclin E–associated cdk2 kinase activity is increased after p27-immunodepletion (ID) of E-cadherin–arrested cells. The representative control in this experiment was Ecad7 lysate immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. In each of these experiments cells were cultured for 48 h in three-dimensional culture.
Figure 5
Increased p27 binding to cyclin E mediates reduced Cyclin E–associated kinase activity in aggregated cells. (a) Cyclin E–associated cdk2 kinase activity, as measured by phosphorylation of Histone H1 substrate in representative neomycin-resistant control transfectants (neo1, neo4, neo8) or E-cadherin transfectants (Ecad1, Ecad9, Ecad18) grown in three-dimensional culture. Also included are Pc5-T cells grown in monolayer (2-D) culture, or in three-dimensional culture in either the presence or absence of hyaluronidase (Hy). (b) Increased levels of p27 bound to cyclin E in tightly adherent EMT/6 spheroids. After immunoprecipitating cyclin E from tightly- or loosely adherent aggregates, levels of cyclin E–bound p27 were detected by immunoblotting. Cell-free lysis buffer was used for the control sample. (c) Immunodepletion of p27 from aggregated E-cadherin transfectants. Cell extracts immunodepleted one (1×) or two (2×) times with antibodies against p27 were probed for p27 by immunoblotting. Controls (Con) represent extracts before immunodepletion (d). Cyclin E, cyclin E–associated cdk2, and p27 are markedly reduced in p27-immunodepleted (ID) supernatents from E-cadherin–aggregated cells. Cyclin E immunoprecipitates from lysates with or without prior immunodepletion with p27 antibodies were probed for cyclin E, cdk2, or p27 by immunoblotting. As a control, Ecad9 lysates were immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. (e) Cyclin E–associated cdk2 kinase activity is increased after p27-immunodepletion (ID) of E-cadherin–arrested cells. The representative control in this experiment was Ecad7 lysate immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. In each of these experiments cells were cultured for 48 h in three-dimensional culture.
Figure 5
Increased p27 binding to cyclin E mediates reduced Cyclin E–associated kinase activity in aggregated cells. (a) Cyclin E–associated cdk2 kinase activity, as measured by phosphorylation of Histone H1 substrate in representative neomycin-resistant control transfectants (neo1, neo4, neo8) or E-cadherin transfectants (Ecad1, Ecad9, Ecad18) grown in three-dimensional culture. Also included are Pc5-T cells grown in monolayer (2-D) culture, or in three-dimensional culture in either the presence or absence of hyaluronidase (Hy). (b) Increased levels of p27 bound to cyclin E in tightly adherent EMT/6 spheroids. After immunoprecipitating cyclin E from tightly- or loosely adherent aggregates, levels of cyclin E–bound p27 were detected by immunoblotting. Cell-free lysis buffer was used for the control sample. (c) Immunodepletion of p27 from aggregated E-cadherin transfectants. Cell extracts immunodepleted one (1×) or two (2×) times with antibodies against p27 were probed for p27 by immunoblotting. Controls (Con) represent extracts before immunodepletion (d). Cyclin E, cyclin E–associated cdk2, and p27 are markedly reduced in p27-immunodepleted (ID) supernatents from E-cadherin–aggregated cells. Cyclin E immunoprecipitates from lysates with or without prior immunodepletion with p27 antibodies were probed for cyclin E, cdk2, or p27 by immunoblotting. As a control, Ecad9 lysates were immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. (e) Cyclin E–associated cdk2 kinase activity is increased after p27-immunodepletion (ID) of E-cadherin–arrested cells. The representative control in this experiment was Ecad7 lysate immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. In each of these experiments cells were cultured for 48 h in three-dimensional culture.
Figure 5
Increased p27 binding to cyclin E mediates reduced Cyclin E–associated kinase activity in aggregated cells. (a) Cyclin E–associated cdk2 kinase activity, as measured by phosphorylation of Histone H1 substrate in representative neomycin-resistant control transfectants (neo1, neo4, neo8) or E-cadherin transfectants (Ecad1, Ecad9, Ecad18) grown in three-dimensional culture. Also included are Pc5-T cells grown in monolayer (2-D) culture, or in three-dimensional culture in either the presence or absence of hyaluronidase (Hy). (b) Increased levels of p27 bound to cyclin E in tightly adherent EMT/6 spheroids. After immunoprecipitating cyclin E from tightly- or loosely adherent aggregates, levels of cyclin E–bound p27 were detected by immunoblotting. Cell-free lysis buffer was used for the control sample. (c) Immunodepletion of p27 from aggregated E-cadherin transfectants. Cell extracts immunodepleted one (1×) or two (2×) times with antibodies against p27 were probed for p27 by immunoblotting. Controls (Con) represent extracts before immunodepletion (d). Cyclin E, cyclin E–associated cdk2, and p27 are markedly reduced in p27-immunodepleted (ID) supernatents from E-cadherin–aggregated cells. Cyclin E immunoprecipitates from lysates with or without prior immunodepletion with p27 antibodies were probed for cyclin E, cdk2, or p27 by immunoblotting. As a control, Ecad9 lysates were immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. (e) Cyclin E–associated cdk2 kinase activity is increased after p27-immunodepletion (ID) of E-cadherin–arrested cells. The representative control in this experiment was Ecad7 lysate immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. In each of these experiments cells were cultured for 48 h in three-dimensional culture.
Figure 5
Increased p27 binding to cyclin E mediates reduced Cyclin E–associated kinase activity in aggregated cells. (a) Cyclin E–associated cdk2 kinase activity, as measured by phosphorylation of Histone H1 substrate in representative neomycin-resistant control transfectants (neo1, neo4, neo8) or E-cadherin transfectants (Ecad1, Ecad9, Ecad18) grown in three-dimensional culture. Also included are Pc5-T cells grown in monolayer (2-D) culture, or in three-dimensional culture in either the presence or absence of hyaluronidase (Hy). (b) Increased levels of p27 bound to cyclin E in tightly adherent EMT/6 spheroids. After immunoprecipitating cyclin E from tightly- or loosely adherent aggregates, levels of cyclin E–bound p27 were detected by immunoblotting. Cell-free lysis buffer was used for the control sample. (c) Immunodepletion of p27 from aggregated E-cadherin transfectants. Cell extracts immunodepleted one (1×) or two (2×) times with antibodies against p27 were probed for p27 by immunoblotting. Controls (Con) represent extracts before immunodepletion (d). Cyclin E, cyclin E–associated cdk2, and p27 are markedly reduced in p27-immunodepleted (ID) supernatents from E-cadherin–aggregated cells. Cyclin E immunoprecipitates from lysates with or without prior immunodepletion with p27 antibodies were probed for cyclin E, cdk2, or p27 by immunoblotting. As a control, Ecad9 lysates were immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. (e) Cyclin E–associated cdk2 kinase activity is increased after p27-immunodepletion (ID) of E-cadherin–arrested cells. The representative control in this experiment was Ecad7 lysate immunoprecipitated with nonspecific rabbit IgG antibodies instead of cyclin E antibodies. In each of these experiments cells were cultured for 48 h in three-dimensional culture.
Figure 6
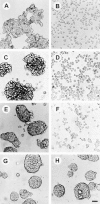
Morphology of human E-cadherin–positive cell lines in three-dimensional culture. E-cadherin positive HT29 colon (a and b), L23 lung (c and d), BT20 breast (e and f), or E-cadherin–negative HBL100 breast carcinoma cell lines (g and h) were cultured in the presence of 2 μg/ml SHE78-7 anti-E-cadherin antibody (b, d, f, and h) or isotype-matched control IgG antibody (a, c, e, and g). Cells were photographed 24 h after plating. Bar, 50 μm.
Figure 8
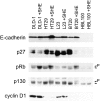
Changes in cell cycle regulators in response to E-cadherin–mediated adhesion of human carcinoma cell lines. Immunoblots of colon (HT29, DLD-1), lung (L23), and breast (BT20) carcinoma cell lines were probed for p27, pRb, p130, and cyclin D1. E-cadherin–dependent intercellular adhesion was prevented by treatment with 2 μg/ml of SHE78-7 antibody (+SHE).
Figure 7
E-cadherin–neutralizing antibodies stimulate the growth of human E-cadherin positive cell lines in three-dimensional culture. Colon (HT29, DLD-1), lung (L23), and breast (BT20, MCF-7, HBL100) carcinoma cell lines were cultured for 48 h in the presence of SHE78-7 anti-E-cadherin antibodies (•) or isotype-matched control IgG antibodies (▴). After pulsing with [3H]thymidine, radioactivity incorporated into DNA was measured and expressed as a percentage of nontreated control cells. Note that E-cadherin antibodies only stimulate the growth of cell lines that display E-cadherin–mediated intercellular adhesion.
Figure 9
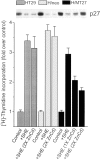
Enforced expression of p27 inhibits cell growth in the presence of E-cadherin neutralizing antibodies. HT29 cells transfected with a metallothionein- inducible p27 expression plasmid (H/MT27), neomycin vector alone (H/neo), or nontransfected HT29 cells (HT29) were grown in three-dimensional culture for 48 h in the absence (Control) or presence of SHE78-7 antibody (+SHE). For induction of the metallothionein promoter, cultures were supplemented with either a one (1×) or two times (2×) concentration of Zinc and Cadmium (ZnCd). After pulsing with [3H]thymidine, radioactivity incorporated into DNA was measured and expressed as fold increase in counts over nontreated control cells. The corresponding levels of p27 as determined by immunoblotting are shown in the inset.
Figure 10
E-cadherin inhibits growth factor–mediated downregulation of p27 through a phosphatase-dependent mechanism. (a) Mitogens are required for growth stimulation in the presence of E-cadherin–neutralizing antibodies. Nontreated HT29 cells (control) were grown in serum-free medium for 48 h. Treated cells were grown in the presence of 2 μg/ml SHE78-7 antibody (+SHE), 10% FCS (+FCS), or 50 ng/ml TGF-α (+_TGF_-α), either alone or in various combinations. To prevent TGF-α activity, cultures were treated with c225-neutralizing anti-EGRF antibody. All factors were added to three-dimensional cell cultures at the time of cell plating. After pulsing with [3H]thymidine, radioactivity incorporated into DNA was measured and expressed as fold increase in counts over nontreated control cells. The corresponding levels of p27 as determined by immunoblotting are shown in the inset. (b) Inhibition of phosphatase activity lowers p27 levels in aggregated HT29 cells. HT29 cells plated in three-dimensional culture in the presence of either FCS or TGF-α were treated with 50 μM sodium orthovanadate (+Van). 48 h later, cells were collected and levels of p27 were determined by immunoblotting.
Figure 10
E-cadherin inhibits growth factor–mediated downregulation of p27 through a phosphatase-dependent mechanism. (a) Mitogens are required for growth stimulation in the presence of E-cadherin–neutralizing antibodies. Nontreated HT29 cells (control) were grown in serum-free medium for 48 h. Treated cells were grown in the presence of 2 μg/ml SHE78-7 antibody (+SHE), 10% FCS (+FCS), or 50 ng/ml TGF-α (+_TGF_-α), either alone or in various combinations. To prevent TGF-α activity, cultures were treated with c225-neutralizing anti-EGRF antibody. All factors were added to three-dimensional cell cultures at the time of cell plating. After pulsing with [3H]thymidine, radioactivity incorporated into DNA was measured and expressed as fold increase in counts over nontreated control cells. The corresponding levels of p27 as determined by immunoblotting are shown in the inset. (b) Inhibition of phosphatase activity lowers p27 levels in aggregated HT29 cells. HT29 cells plated in three-dimensional culture in the presence of either FCS or TGF-α were treated with 50 μM sodium orthovanadate (+Van). 48 h later, cells were collected and levels of p27 were determined by immunoblotting.
Figure 11
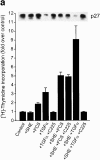
Inhibiting PI3K activity or generating reactive oxygen species elevates p27 protein levels and reduces [3H]thymidine uptake of E-cadherin antibody-dispersed HT29 cells. Cells plated into three-dimensional cuture in the presence of TGF-α and SHE78-7 antibody were treated with either (a) the PI3K inhibitor LY294002 (LY) or (b) the antioxidant _N_-acetyl-
l
-cysteine (NAC). [3H]thymidine incorporation was measured after 24 h. The inset for both a and b shows the corresponding levels of p27 protein as determined by immunoblotting.
References
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. - PubMed
- Alessandrini A, Chiaur DS, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. - PubMed
- Allison DC, Yuhas JM, Ridolpho PF, Anderson SL, Johnson TS. Cytophotometric measurement of the cellular DNA content of [3H]thymidine-labeled spheroids. Demonstration that some non-labeled cells have S and G2 DNA content. Cell Tissue Kinet. 1983;16:237–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous