Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1 - PubMed (original) (raw)
Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1
I B Mazo et al. J Exp Med. 1998.
Erratum in
- J Exp Med 1998 Sep 7;188(5):1001
Abstract
We have used intravital microscopy to study physiologically perfused microvessels in murine bone marrow (BM). BM sinusoids and venules, but not adjacent bone vessels, supported rolling interactions of hematopoietic progenitor cells. Rolling did not involve L-selectin, but was partially reduced in wild-type mice treated with antibodies to P- or E-selectin and in mice that were deficient in these two selectins. Selectin-independent rolling was mediated by alpha4 integrins, which interacted with endothelial vascular cell adhesion molecule (VCAM)-1. Parallel contribution of the endothelial selectins and VCAM-1 is not known to direct blood cell trafficking to other noninflamed tissues. This combination of constitutively expressed adhesion molecules may thus constitute a BM-specific recruitment pathway for progenitor cells analogous to the vascular addressins that direct selective lymphocyte homing to lymphoid organs.
Figures
Figure 1
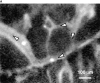
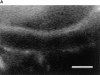
Anatomy and histology of murine skull BM. (A) Top view of the dorsal murine skull. Major flat bones and sutures are shown schematically (left). The rectangle over the frontoparietal region indicates the area which shows the typical microvascular anatomy after intravenous injection of 150-kD FITC-dextran (right). A large BMCV (1) in the parietal bone parallel to the coronal suture drains into the sagittal sinus (2). A parietal network of sinusoids (3) drains into the BMCV, either directly or via one or more BMPSV (4). BMCVs also receive blood from laterally located venules in the parietal bone. These vessels include BMIVs (5) and some BVs (6). A dense parasagittal CN (7) is located in the frontal bone. Blood flow in CN capillaries is highly variable and often partially obscured by red marrow, which tends to be more concentrated in this area than elsewhere. (B) Rhodamine 6G accumulation. A marked accumulation of the dye (R) is observed in the parasagittal regions of frontal and parietal bones and in the perivascular space of sinusoids and some, but not all, venules. Identical staining patterns were seen with rhodamine 123 (data not shown). Number designations are as in A. (C) Photomicrographs of hematoxylin and eosin–stained frontal sections through the frontal bones of a skull. The sections lie approximately on the long axis of the quadrangle in A (left). Several intraosseous cavities filled with densely packed hematopoietic cells are shown at low magnification (top). Arrows indicate the sagittal (left) and coronal (right) sutures; SS, sagittal sinus. At higher magnification (bottom), a microvessel (*) can be detected within a BM cavity. Scale bars in top and lower panels depict 500 and 50 μm, respectively. (D) Colony-forming capacity of MNCs from murine brain, skull, and femur. MNCs isolated from brains, skullcaps, and femora of five mice were harvested and plated on methylcellulose. CFU-C assays were performed in duplicate. Data are expressed as mean ± SEM of three independent experiments.
Figure 1
Anatomy and histology of murine skull BM. (A) Top view of the dorsal murine skull. Major flat bones and sutures are shown schematically (left). The rectangle over the frontoparietal region indicates the area which shows the typical microvascular anatomy after intravenous injection of 150-kD FITC-dextran (right). A large BMCV (1) in the parietal bone parallel to the coronal suture drains into the sagittal sinus (2). A parietal network of sinusoids (3) drains into the BMCV, either directly or via one or more BMPSV (4). BMCVs also receive blood from laterally located venules in the parietal bone. These vessels include BMIVs (5) and some BVs (6). A dense parasagittal CN (7) is located in the frontal bone. Blood flow in CN capillaries is highly variable and often partially obscured by red marrow, which tends to be more concentrated in this area than elsewhere. (B) Rhodamine 6G accumulation. A marked accumulation of the dye (R) is observed in the parasagittal regions of frontal and parietal bones and in the perivascular space of sinusoids and some, but not all, venules. Identical staining patterns were seen with rhodamine 123 (data not shown). Number designations are as in A. (C) Photomicrographs of hematoxylin and eosin–stained frontal sections through the frontal bones of a skull. The sections lie approximately on the long axis of the quadrangle in A (left). Several intraosseous cavities filled with densely packed hematopoietic cells are shown at low magnification (top). Arrows indicate the sagittal (left) and coronal (right) sutures; SS, sagittal sinus. At higher magnification (bottom), a microvessel (*) can be detected within a BM cavity. Scale bars in top and lower panels depict 500 and 50 μm, respectively. (D) Colony-forming capacity of MNCs from murine brain, skull, and femur. MNCs isolated from brains, skullcaps, and femora of five mice were harvested and plated on methylcellulose. CFU-C assays were performed in duplicate. Data are expressed as mean ± SEM of three independent experiments.
Figure 1
Anatomy and histology of murine skull BM. (A) Top view of the dorsal murine skull. Major flat bones and sutures are shown schematically (left). The rectangle over the frontoparietal region indicates the area which shows the typical microvascular anatomy after intravenous injection of 150-kD FITC-dextran (right). A large BMCV (1) in the parietal bone parallel to the coronal suture drains into the sagittal sinus (2). A parietal network of sinusoids (3) drains into the BMCV, either directly or via one or more BMPSV (4). BMCVs also receive blood from laterally located venules in the parietal bone. These vessels include BMIVs (5) and some BVs (6). A dense parasagittal CN (7) is located in the frontal bone. Blood flow in CN capillaries is highly variable and often partially obscured by red marrow, which tends to be more concentrated in this area than elsewhere. (B) Rhodamine 6G accumulation. A marked accumulation of the dye (R) is observed in the parasagittal regions of frontal and parietal bones and in the perivascular space of sinusoids and some, but not all, venules. Identical staining patterns were seen with rhodamine 123 (data not shown). Number designations are as in A. (C) Photomicrographs of hematoxylin and eosin–stained frontal sections through the frontal bones of a skull. The sections lie approximately on the long axis of the quadrangle in A (left). Several intraosseous cavities filled with densely packed hematopoietic cells are shown at low magnification (top). Arrows indicate the sagittal (left) and coronal (right) sutures; SS, sagittal sinus. At higher magnification (bottom), a microvessel (*) can be detected within a BM cavity. Scale bars in top and lower panels depict 500 and 50 μm, respectively. (D) Colony-forming capacity of MNCs from murine brain, skull, and femur. MNCs isolated from brains, skullcaps, and femora of five mice were harvested and plated on methylcellulose. CFU-C assays were performed in duplicate. Data are expressed as mean ± SEM of three independent experiments.
Figure 1
Anatomy and histology of murine skull BM. (A) Top view of the dorsal murine skull. Major flat bones and sutures are shown schematically (left). The rectangle over the frontoparietal region indicates the area which shows the typical microvascular anatomy after intravenous injection of 150-kD FITC-dextran (right). A large BMCV (1) in the parietal bone parallel to the coronal suture drains into the sagittal sinus (2). A parietal network of sinusoids (3) drains into the BMCV, either directly or via one or more BMPSV (4). BMCVs also receive blood from laterally located venules in the parietal bone. These vessels include BMIVs (5) and some BVs (6). A dense parasagittal CN (7) is located in the frontal bone. Blood flow in CN capillaries is highly variable and often partially obscured by red marrow, which tends to be more concentrated in this area than elsewhere. (B) Rhodamine 6G accumulation. A marked accumulation of the dye (R) is observed in the parasagittal regions of frontal and parietal bones and in the perivascular space of sinusoids and some, but not all, venules. Identical staining patterns were seen with rhodamine 123 (data not shown). Number designations are as in A. (C) Photomicrographs of hematoxylin and eosin–stained frontal sections through the frontal bones of a skull. The sections lie approximately on the long axis of the quadrangle in A (left). Several intraosseous cavities filled with densely packed hematopoietic cells are shown at low magnification (top). Arrows indicate the sagittal (left) and coronal (right) sutures; SS, sagittal sinus. At higher magnification (bottom), a microvessel (*) can be detected within a BM cavity. Scale bars in top and lower panels depict 500 and 50 μm, respectively. (D) Colony-forming capacity of MNCs from murine brain, skull, and femur. MNCs isolated from brains, skullcaps, and femora of five mice were harvested and plated on methylcellulose. CFU-C assays were performed in duplicate. Data are expressed as mean ± SEM of three independent experiments.
Figure 2
FDCP-mix cells roll in BM venules and sinusoids. (A) Micrograph of a segment of the right parietal sinusoidal network. Blood flow in some vessels is indicated by arrowheads. To improve contrast between the intra- and extravascular compartment, this animal was injected intravenously with low-dose FITC-dextran (150 kD, 10 mg/g body weight). Three BCECF-labeled FDCP-mix cells (bright intravascular dots) can be seen. (B) Comparison of FDCP-mix cell rolling fractions in BM and bone venules. Rolling fractions in all types of BM venules and sinusoids were significantly higher than in BVs (Student's two-tailed t test for unpaired data). Therefore, data from BM vessels were pooled for this figure. Data are shown as mean ± SEM. n, number of venules. (C) Role of selectins in FDCP-mix cell rolling in wild-type mice. FDCP-mix cell rolling was measured in the same BM vessels before and after injection of anti–P- and/or anti–E-selectin mAbs (100 μg/mouse) or treatment of cells with anti– L-selectin mAb (100 μg/107 cells). Each bar represents the mean ± SEM of three experiments. n corresponds to the number of venules. Groups were compared with control using the Kruskal-Wallis test followed by Bonferoni correction of P. (D) Comparison of FDCP-mix cell rolling in wild-type and P/E-selectin double knockout mice. Data are expressed as mean ± SEM of three experiments. Rolling fractions in P/E-deficient mice were significantly lower than in wild-type littermates (P <0.001; Student's t test), but were not affected by anti–L-selectin mAb (100 μg/107 cells). n, number of venules.
Figure 2
FDCP-mix cells roll in BM venules and sinusoids. (A) Micrograph of a segment of the right parietal sinusoidal network. Blood flow in some vessels is indicated by arrowheads. To improve contrast between the intra- and extravascular compartment, this animal was injected intravenously with low-dose FITC-dextran (150 kD, 10 mg/g body weight). Three BCECF-labeled FDCP-mix cells (bright intravascular dots) can be seen. (B) Comparison of FDCP-mix cell rolling fractions in BM and bone venules. Rolling fractions in all types of BM venules and sinusoids were significantly higher than in BVs (Student's two-tailed t test for unpaired data). Therefore, data from BM vessels were pooled for this figure. Data are shown as mean ± SEM. n, number of venules. (C) Role of selectins in FDCP-mix cell rolling in wild-type mice. FDCP-mix cell rolling was measured in the same BM vessels before and after injection of anti–P- and/or anti–E-selectin mAbs (100 μg/mouse) or treatment of cells with anti– L-selectin mAb (100 μg/107 cells). Each bar represents the mean ± SEM of three experiments. n corresponds to the number of venules. Groups were compared with control using the Kruskal-Wallis test followed by Bonferoni correction of P. (D) Comparison of FDCP-mix cell rolling in wild-type and P/E-selectin double knockout mice. Data are expressed as mean ± SEM of three experiments. Rolling fractions in P/E-deficient mice were significantly lower than in wild-type littermates (P <0.001; Student's t test), but were not affected by anti–L-selectin mAb (100 μg/107 cells). n, number of venules.
Figure 2
FDCP-mix cells roll in BM venules and sinusoids. (A) Micrograph of a segment of the right parietal sinusoidal network. Blood flow in some vessels is indicated by arrowheads. To improve contrast between the intra- and extravascular compartment, this animal was injected intravenously with low-dose FITC-dextran (150 kD, 10 mg/g body weight). Three BCECF-labeled FDCP-mix cells (bright intravascular dots) can be seen. (B) Comparison of FDCP-mix cell rolling fractions in BM and bone venules. Rolling fractions in all types of BM venules and sinusoids were significantly higher than in BVs (Student's two-tailed t test for unpaired data). Therefore, data from BM vessels were pooled for this figure. Data are shown as mean ± SEM. n, number of venules. (C) Role of selectins in FDCP-mix cell rolling in wild-type mice. FDCP-mix cell rolling was measured in the same BM vessels before and after injection of anti–P- and/or anti–E-selectin mAbs (100 μg/mouse) or treatment of cells with anti– L-selectin mAb (100 μg/107 cells). Each bar represents the mean ± SEM of three experiments. n corresponds to the number of venules. Groups were compared with control using the Kruskal-Wallis test followed by Bonferoni correction of P. (D) Comparison of FDCP-mix cell rolling in wild-type and P/E-selectin double knockout mice. Data are expressed as mean ± SEM of three experiments. Rolling fractions in P/E-deficient mice were significantly lower than in wild-type littermates (P <0.001; Student's t test), but were not affected by anti–L-selectin mAb (100 μg/107 cells). n, number of venules.
Figure 2
FDCP-mix cells roll in BM venules and sinusoids. (A) Micrograph of a segment of the right parietal sinusoidal network. Blood flow in some vessels is indicated by arrowheads. To improve contrast between the intra- and extravascular compartment, this animal was injected intravenously with low-dose FITC-dextran (150 kD, 10 mg/g body weight). Three BCECF-labeled FDCP-mix cells (bright intravascular dots) can be seen. (B) Comparison of FDCP-mix cell rolling fractions in BM and bone venules. Rolling fractions in all types of BM venules and sinusoids were significantly higher than in BVs (Student's two-tailed t test for unpaired data). Therefore, data from BM vessels were pooled for this figure. Data are shown as mean ± SEM. n, number of venules. (C) Role of selectins in FDCP-mix cell rolling in wild-type mice. FDCP-mix cell rolling was measured in the same BM vessels before and after injection of anti–P- and/or anti–E-selectin mAbs (100 μg/mouse) or treatment of cells with anti– L-selectin mAb (100 μg/107 cells). Each bar represents the mean ± SEM of three experiments. n corresponds to the number of venules. Groups were compared with control using the Kruskal-Wallis test followed by Bonferoni correction of P. (D) Comparison of FDCP-mix cell rolling in wild-type and P/E-selectin double knockout mice. Data are expressed as mean ± SEM of three experiments. Rolling fractions in P/E-deficient mice were significantly lower than in wild-type littermates (P <0.001; Student's t test), but were not affected by anti–L-selectin mAb (100 μg/107 cells). n, number of venules.
Figure 3
The α4/VCAM-1 pathway mediates selectin-independent rolling of progenitor cells in BM microvessels. (A) CFSE-labeled anti–VCAM-1 accumulation in a typical BM venule. 50 μg of fluorescently labeled mAb MK 2.7 was injected intravenously. 15 min later, the animal was anesthetized, the vena cava was cut, and the mouse was perfused through a catheter in the right common carotid artery with heparinized ice-cold saline to remove intravascular blood and unbound mAb. The optical plane was focused on the centerline of the venule revealing fluorescent mAb at the luminal surface of opposing vessel walls (upward curved horizontal lines). Scale bar depicts 50 μm. (B and C) Effect of mAbs against integrins and VCAM-1 in (B) wild-type mice and (C) P/E-selectin double knockout mice. Rolling fractions after mAb treatment were normalized to rolling fractions determined in the same vessel before mAb application (control). Only FDCP-mix cells were tested in P/E-selectin knockout animals. Groups were compared with control using the Kruskal-Wallis test with Bonferoni correction of P (for FDCP-mix cells) or the Student's two-tailed t test for paired data (for M1 cells). All data are shown as mean ± SEM of three independent experiments. n, number of venules per group.
Figure 3
The α4/VCAM-1 pathway mediates selectin-independent rolling of progenitor cells in BM microvessels. (A) CFSE-labeled anti–VCAM-1 accumulation in a typical BM venule. 50 μg of fluorescently labeled mAb MK 2.7 was injected intravenously. 15 min later, the animal was anesthetized, the vena cava was cut, and the mouse was perfused through a catheter in the right common carotid artery with heparinized ice-cold saline to remove intravascular blood and unbound mAb. The optical plane was focused on the centerline of the venule revealing fluorescent mAb at the luminal surface of opposing vessel walls (upward curved horizontal lines). Scale bar depicts 50 μm. (B and C) Effect of mAbs against integrins and VCAM-1 in (B) wild-type mice and (C) P/E-selectin double knockout mice. Rolling fractions after mAb treatment were normalized to rolling fractions determined in the same vessel before mAb application (control). Only FDCP-mix cells were tested in P/E-selectin knockout animals. Groups were compared with control using the Kruskal-Wallis test with Bonferoni correction of P (for FDCP-mix cells) or the Student's two-tailed t test for paired data (for M1 cells). All data are shown as mean ± SEM of three independent experiments. n, number of venules per group.
Figure 3
The α4/VCAM-1 pathway mediates selectin-independent rolling of progenitor cells in BM microvessels. (A) CFSE-labeled anti–VCAM-1 accumulation in a typical BM venule. 50 μg of fluorescently labeled mAb MK 2.7 was injected intravenously. 15 min later, the animal was anesthetized, the vena cava was cut, and the mouse was perfused through a catheter in the right common carotid artery with heparinized ice-cold saline to remove intravascular blood and unbound mAb. The optical plane was focused on the centerline of the venule revealing fluorescent mAb at the luminal surface of opposing vessel walls (upward curved horizontal lines). Scale bar depicts 50 μm. (B and C) Effect of mAbs against integrins and VCAM-1 in (B) wild-type mice and (C) P/E-selectin double knockout mice. Rolling fractions after mAb treatment were normalized to rolling fractions determined in the same vessel before mAb application (control). Only FDCP-mix cells were tested in P/E-selectin knockout animals. Groups were compared with control using the Kruskal-Wallis test with Bonferoni correction of P (for FDCP-mix cells) or the Student's two-tailed t test for paired data (for M1 cells). All data are shown as mean ± SEM of three independent experiments. n, number of venules per group.
Figure 4
Fluorescently labeled HPC from day 11 FLs of C57/B6J embryos were analyzed in BM microvessels of wild-type and P/E-selectin–deficient mice before and after treatment with anti–VCAM-1 (100 μg/mouse). Data in wild-type mice are from three independent experiments. FL HPC rolling was determined in three P/E-selectin-deficient mice, and one of these animals was additionally treated with anti–VCAM-1. All bars depict mean ± SEM. n, number of venules. Groups were compared using Student's t test for paired or unpaired data where appropriate.
Similar articles
- Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow.
Mazo IB, Quackenbush EJ, Lowe JB, von Andrian UH. Mazo IB, et al. Blood. 2002 Jun 1;99(11):4182-91. doi: 10.1182/blood.v99.11.4182. Blood. 2002. PMID: 12010824 - Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow.
Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Frenette PS, et al. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14423-8. doi: 10.1073/pnas.95.24.14423. Proc Natl Acad Sci U S A. 1998. PMID: 9826716 Free PMC article. - Overlapping roles of endothelial selectins and vascular cell adhesion molecule-1 in immune complex-induced leukocyte recruitment in the cremasteric microvasculature.
Norman MU, Van De Velde NC, Timoshanko JR, Issekutz A, Hickey MJ. Norman MU, et al. Am J Pathol. 2003 Oct;163(4):1491-503. doi: 10.1016/S0002-9440(10)63506-7. Am J Pathol. 2003. PMID: 14507656 Free PMC article. - New discoveries with mice mutant in endothelial and platelet selectins.
Hartwell DW, Wagner DD. Hartwell DW, et al. Thromb Haemost. 1999 Aug;82(2):850-7. Thromb Haemost. 1999. PMID: 10605793 Review. - Adhesion and homing of blood-borne cells in bone marrow microvessels.
Mazo IB, von Andrian UH. Mazo IB, et al. J Leukoc Biol. 1999 Jul;66(1):25-32. doi: 10.1002/jlb.66.1.25. J Leukoc Biol. 1999. PMID: 10410986 Review.
Cited by
- Tracking mouse bone marrow monocytes in vivo.
Hamon P, Rodero MP, Combadière C, Boissonnas A. Hamon P, et al. J Vis Exp. 2015 Feb 27;(96):e52476. doi: 10.3791/52476. J Vis Exp. 2015. PMID: 25867540 Free PMC article. - High-resolution imaging and computational analysis of haematopoietic cell dynamics in vivo.
Koechlein CS, Harris JR, Lee TK, Weeks J, Fox RG, Zimdahl B, Ito T, Blevins A, Jung SH, Chute JP, Chourasia A, Covert MW, Reya T. Koechlein CS, et al. Nat Commun. 2016 Jul 18;7:12169. doi: 10.1038/ncomms12169. Nat Commun. 2016. PMID: 27425143 Free PMC article. - The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils.
Henderson RB, Lim LH, Tessier PA, Gavins FN, Mathies M, Perretti M, Hogg N. Henderson RB, et al. J Exp Med. 2001 Jul 16;194(2):219-26. doi: 10.1084/jem.194.2.219. J Exp Med. 2001. PMID: 11457896 Free PMC article. - Collagen-based cell migration models in vitro and in vivo.
Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Wolf K, et al. Semin Cell Dev Biol. 2009 Oct;20(8):931-41. doi: 10.1016/j.semcdb.2009.08.005. Epub 2009 Aug 12. Semin Cell Dev Biol. 2009. PMID: 19682592 Free PMC article. Review. - Defects in osteoblast function but no changes in long-term repopulating potential of hematopoietic stem cells in a mouse chronic inflammatory arthritis model.
Ma YD, Park C, Zhao H, Oduro KA Jr, Tu X, Long F, Allen PM, Teitelbaum SL, Choi K. Ma YD, et al. Blood. 2009 Nov 12;114(20):4402-10. doi: 10.1182/blood-2008-12-196311. Epub 2009 Sep 16. Blood. 2009. PMID: 19759358 Free PMC article.
References
- Friedrich C, Cybulsky MI, Gutierrez-Ramos JC. Vascular cell adhesion molecule-1 expression by hematopoiesis-supporting stromal cells is not essential for lymphoid or myeloid differentiation in vivo or in vitro. Eur J Immunol. 1996;26:2773–2780. - PubMed
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell. 1994;76:301–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous