MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta - PubMed (original) (raw)
MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta
F S Lee et al. Proc Natl Acad Sci U S A. 1998.
Abstract
A critical step in the signal-induced activation of the transcription factor NF-kappaB is the site-specific phosphorylation of its inhibitor, IkappaB, that targets the latter for degradation by the ubiquitin-proteasome pathway. We have previously shown that mitogen-activated protein kinase/ERK kinase kinase 1 (MEKK1) can induce both this site-specific phosphorylation of IkappaB alpha at Ser-32 and Ser-36 in vivo and the activity of a high molecular weight IkappaB kinase complex in vitro. Subsequently, others have identified two proteins, IkappaB kinase alpha (IKK-alpha) and IkappaB kinase beta (IKK-beta), that are present in a tumor necrosis factor alpha-inducible, high molecular weight IkappaB kinase complex. These kinases are believed to directly phosphorylate IkappaB based on the examination of the kinase activities of IKK immunoprecipitates, but more rigorous proof of this has yet to be demonstrated. We show herein that recombinant IKK-alpha and IKK-beta can, in fact, directly phosphorylate IkappaB alpha at Ser-32 and Ser-36, as well as homologous residues in IkappaB beta in vitro, and thus are bona fide IkappaB kinases. We also show that MEKK1 can induce the activation of both IKK-alpha and IKK-beta in vivo. Finally, we show that IKK-alpha is present in the MEKK1-inducible, high molecular weight IkappaB kinase complex and treatment of this complex with MEKK1 induces phosphorylation of IKK-alpha in vitro. We conclude that IKK-alpha and IKK-beta can mediate the NF-kappaB-inducing activity of MEKK1.
Figures
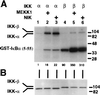
Figure 1
Phosphorylation of IκBα and IκBβ by recombinant IKK-α and IKK-β. (His)6-tagged IKK-α (A) and IKK-β (A and B) were purified from baculovirus-infected Sf9 cells with Ni-NTA agarose chromatography (A) and further by Mono Q chromatography (B), and 500 (A) or 20 ng (B) was subjected to SDS/PAGE in 8 or 10% gels and stained with Coomassie blue (A) or silver (B). An asterisk in B denotes a polypeptide copurifying with IKK-β. (C–E) GST-IκBα-(5–55) (0.5 μg) or GST-IκBβ-(1–46) (0.5 μg) bearing the indicated residues was incubated in the presence of [γ-32P]ATP with recombinant IKK-α (10 ng) (C) or IKK-β (1 ng) (D and E) purified by Ni-agarose chromatography (C and D) or further by Mono Q chromatography (E). The first and second residues correspond to positions 32 or 36 of IκBα, respectively, or to positions 19 and 23 of IκBβ, respectively. Reaction products were subjected to SDS/PAGE in 10% gels and analyzed by autoradiography. (F) (His)6IκBα (0.5 μg), (His)6IκBα (S32A/S36A) (0.5 μg), (His)6IκBβ (0.5 μg), or (His)6IκBβ (S19A/S23A) (0.5 μg) was incubated in the presence of [γ-32P]ATP with 10 ng of recombinant IKK-α or IKK-β purified by Ni-agarose chromatography. Reaction products were subjected to SDS/PAGE in 9% gels and analyzed by autoradiography. The slower migration of wild-type as compared with S32A/S36A (His)6IκBα reflects phosphorylation at Ser-32 and -36 (6). (A–F) Molecular mass markers (in kDa) are indicated to the left, and the positions of IKK-α, IKK-β, and the IκBα and IκBβ substrates are indicated to the right. The relative levels of 32P incorporation into the IκB substrates are indicated below the gels.
Figure 2
Activation of IKK-α and IKK-β by MEKK1 and NIK. HeLa cells were transfected with 1 μg of pRK-FlagIKK-α or 1 μg of pRK-FlagIKK-β and 6 μg of pCMV5-MEKK1, 6 μg of pcDNA3-NIK, or 6 μg of pcDNA3. Cells were harvested 42 hr after transfection, and IKK-α or IKK-β was immunoprecipitated with anti-Flag M2-agarose. (A) IKK immunocomplexes were assayed for kinase activity by incubation with 0.5 μg of GST-IκBα-(5–55) in the presence of [γ-32P]ATP. Reaction products were subjected to SDS/PAGE in 10% gels and analyzed by autoradiography. The relative level of 32P incorporation into the GST-IκBα-(5–55) substrate is indicated below the gel. (B) IKK immunocomplexes were subjected to SDS/PAGE in 8% gels, transferred to nitrocellulose membrane, and probed with anti-Flag polyclonal antibodies. (A and B) The positions of IKK-α, IKK-β, and GST-IκBα-(5–55) are indicated to the left, and molecular mass markers (in kDa) are indicated to the right.
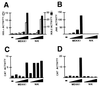
Figure 3
Effects of MEKK1 and NIK on the NF-κB and JNK pathways. HeLa cells were transfected with 2 μg of pCMV-lacZ; 1 μg of pRK-FlagIKK-α (A), 1 μg of pRK-FlagIKK-β (A), 1 μg of pcDNA3-FlagJNK1 (B), 3 μg of (PRDII)2CAT (C), or 3 μg of (PRDIV)6CAT (D); and 5, 50, 500, or 5000 ng of either pCMV5-MEKK1 or pcDNA3-NIK. The total DNA dose was brought up to 8 (A and B) or 10 (C and D) μg with pcDNA3. Cells were harvested 40–42 hr after transfection. (A and B) Immunocomplex kinase assays. Whole cell extracts, the volumes of which were normalized for β-galactosidase activity, were subjected to immunoprecipitation with anti-Flag M2-agarose. Immunocomplexes were assayed for IKK (A) or JNK (B) activities by incubation with GST-IκBα-(5–55) or GST-cJun-(1–79), respectively, in the presence of [γ-32P]ATP. Western blots performed on aliquots of whole cell extracts revealed that comparable amounts of protein kinase were subjected to immunoprecipitation in each series of experiments (data not shown). Activities were normalized to that of IKK-α, IKK-β, or JNK alone, each of which was assigned a value of 1. (C and D) CAT assays. Activities were normalized to protein concentrations of cell extracts. Shown is a representative result from three experiments.
Figure 4
Inhibition of MEKK1- and NIK-induced NF-κB activation by dominant negative IKK-α or dominant negative IKK-β. HeLa cells were transfected with 3 μg of (PRDII)2CAT; 4 μg of pcDNA3-FlagMEKK1Δ, 0.1 μg of pcDNA3-FlagNIK, or 4 μg of pcDNA3; and 4 μg of pRK-FlagIKK-α (K44A), pRK-FlagIKK-β (K44A), or pcDNA3. The total DNA dose was brought up to 11 μg with pcDNA3. Cells were harvested 40 hr after transfection. CAT activities were normalized to protein concentrations of cell extracts. Shown is a representative result from three experiments.
Figure 5
MEKK1-induced phosphorylation of IKK. (A and B) IκBα kinase complex was incubated with or without 40 ng MEKK1Δ, in the absence (A) or presence (B) of [γ-32P]ATP. Reactions were then immunoprecipitated (IP) with either anti-Stat1 or anti-IKK-α antibodies and analyzed by Western blotting (A) using anti-IKK-α antibodies or by autoradiography (B). The position of IKK-α is as indicated and the position of a slower migrating species is denoted by an asterisk (see Results). (C) IκBα kinase complex was incubated with 35S-labeled _in vitro_-translated Flag-IκBα in the absence or presence of 40 ng MEKK1Δ. Reaction products were subjected to SDS/PAGE in 9% gels and detected by autoradiography. The positions of Flag-IκBα (IκBα) and phospho-Flag-IκBα (P-IκBα) are indicated to the left. (D) IKK-β-(171–187) (2 μg) or IKK-β-(171–187) (S177A/S181A) (2 μg) peptide was incubated with or without 100 ng of recombinant MEKK1Δ in the presence of [γ-32P]ATP. Reaction products were subjected to gradient SDS/PAGE in 10–20% gels and analyzed by autoradiography. The positions of molecular mass markers (in kDa) are indicated to the left (B) or right (A, C, and D).
Comment in
- JNK or IKK, AP-1 or NF-kappaB, which are the targets for MEK kinase 1 action?
Karin M, Delhase M. Karin M, et al. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9067-9. doi: 10.1073/pnas.95.16.9067. Proc Natl Acad Sci U S A. 1998. PMID: 9689033 Free PMC article. No abstract available.
Similar articles
- Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway.
Lee FS, Hagler J, Chen ZJ, Maniatis T. Lee FS, et al. Cell. 1997 Jan 24;88(2):213-22. doi: 10.1016/s0092-8674(00)81842-5. Cell. 1997. PMID: 9008162 - Coordinate regulation of IkappaB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-kappaB-inducing kinase.
Nemoto S, DiDonato JA, Lin A. Nemoto S, et al. Mol Cell Biol. 1998 Dec;18(12):7336-43. doi: 10.1128/MCB.18.12.7336. Mol Cell Biol. 1998. PMID: 9819420 Free PMC article. - Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.
Karin M, Ben-Neriah Y. Karin M, et al. Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review. - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
- Induction of chemokines in human astrocytes by picornavirus infection requires activation of both AP-1 and NF-kappa B.
Kwon D, Fuller AC, Palma JP, Choi IH, Kim BS. Kwon D, et al. Glia. 2004 Feb;45(3):287-96. doi: 10.1002/glia.10331. Glia. 2004. PMID: 14730702 Free PMC article. - Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation.
Yeung KC, Rose DW, Dhillon AS, Yaros D, Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Yeung KC, et al. Mol Cell Biol. 2001 Nov;21(21):7207-17. doi: 10.1128/MCB.21.21.7207-7217.2001. Mol Cell Biol. 2001. PMID: 11585904 Free PMC article. - Mixed-lineage kinase 3 delivers CD3/CD28-derived signals into the IkappaB kinase complex.
Hehner SP, Hofmann TG, Ushmorov A, Dienz O, Wing-Lan Leung I, Lassam N, Scheidereit C, Dröge W, Schmitz ML. Hehner SP, et al. Mol Cell Biol. 2000 Apr;20(7):2556-68. doi: 10.1128/MCB.20.7.2556-2568.2000. Mol Cell Biol. 2000. PMID: 10713178 Free PMC article. - Activation of early signaling transcription factor, NF-kappaB following low-level manganese exposure.
Ramesh GT, Ghosh D, Gunasekar PG. Ramesh GT, et al. Toxicol Lett. 2002 Dec 15;136(2):151-8. doi: 10.1016/s0378-4274(02)00332-6. Toxicol Lett. 2002. PMID: 12425965 Free PMC article. - IKK gamma (NEMO) is involved in the coordination of the AP-1 and NF-kappa B pathways.
Shifera AS, Friedman JM, Horwitz MS. Shifera AS, et al. Mol Cell Biochem. 2008 Mar;310(1-2):181-90. doi: 10.1007/s11010-007-9679-z. Epub 2007 Dec 16. Mol Cell Biochem. 2008. PMID: 18080803
References
- Lee F S, Chen Z J, Maniatis T. In: Transcriptional Regulation of Endothelial Cell Adhesion Molecules. Collins T, editor. Austin, TX: Landes; 1998. , in press.
- May M J, Ghosh S. Immunol Today. 1998;19:80–88. - PubMed
- Chen Z J, Hagler J, Palombella V, Melandri F, Scherer D, Ballard D, Maniatis T. Genes Dev. 1995;9:1586–1597. - PubMed
- Maniatis T. Science. 1997;278:818–819. - PubMed
- Stankovski I, Baltimore D. Cell. 1997;91:299–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous