Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice - PubMed (original) (raw)
Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice
Q Ma et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The chemokine stromal cell-derived factor 1, SDF-1, is an important regulator of leukocyte and hematopoietic precursor migration and pre-B cell proliferation. The receptor for SDF-1, CXCR4, also functions as a coreceptor for T-tropic HIV-1 entry. We find that mice deficient for CXCR4 die perinatally and display profound defects in the hematopoietic and nervous systems. CXCR4-deficient mice have severely reduced B-lymphopoiesis, reduced myelopoiesis in fetal liver, and a virtual absence of myelopoiesis in bone marrow. However, T-lymphopoiesis is unaffected. Furthermore, the cerebellum develops abnormally with an irregular external granule cell layer, ectopically located Purkinje cells, and numerous chromophilic cell clumps of abnormally migrated granule cells within the cerebellar anlage. Identical defects are observed in mice lacking SDF-1, suggesting a monogamous relationship between CXCR4 and SDF-1. This receptor-ligand selectivity is unusual among chemokines and their receptors, as is the function in migration of nonhematopoietic cells.
Figures
Figure 1
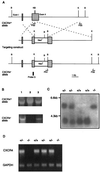
Generation of CXCR4−/− mice. (A) Schematic diagram of the CXCR4 genomic locus, the targeting construct, and the targeted CXCR4 allele. B, _Bam_HI; K, _Kpn_I. Shadowed boxes represent the two coding exons of the CXCR4 gene. The 80-bp _Kpn_I-_Bam_HI fragment of exon II is replaced by the pgk-neomycin-resistance gene cassette (Neor, open box) resulting in disruption of the fourth transmembrane domain of CXCR4-coding sequences. (B) Three representative ES cell clones screened by PCR using PS5, a 3′ common primer, in combination with PS2 for detection of the CXCR4+ allele or PN1 for detection of CXCR4− allele. (C) Southern blots with probe Q on _Bam_HI-digest DNA to genotype representative mutant and littermate mice. (D) Reverse transcription–PCR analysis of brain demonstrates the absence of CXCR4 transcript in CXCR4−/− mice. Glyceraldehyde-3-phosphate dehydrogenase is the control transcript.
Figure 2
Defects in B-lymphopoiesis and myelopoiesis and normal T lymphopoiesis in CXCR4−/− mice determined by flow cytometry. (A and B) Pro-B cells (B220+CD43+) within lymphocyte scatter gates were enumerated in E18.5 fetal liver (A) and 5 day Whitlock–Witte culture with IL-7 of E15.5 fetal liver cells (B). (C) Staining of Gr-1+CD11b+ (Upper) and CD18+CD11b+ (Lower) myeloid cells within scatter gates for myelomonocytic cells in E18.5 fetal liver. E18.5 fetal thymocytes (D) or cells from thymic organ culture of E15.5 thymus (E) were stained with CD4 and CD8 mAb. The percentages of gated cells within the boxes are indicated.
Figure 3
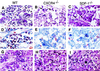
Defects in B-lymphopoiesis and myelopoiesis in CXCR4−/− and SDF-1−/− mice. (A_–_C) Sections of femur bone marrow stained with H&E. Arrows indicate erythroid and megakaryocytic cells. (D_–_F) Sections of bone marrow stained for chloroacetate esterase activity. (G_–_I) Sections of liver stained with H&E.
Figure 4
Cerebellum defects in CXCR4−/− and SDF-1−/− mice. Coronal sections of E18.5 cerebellum stained with H&E. Arrows indicate EGL and asterisk indicates Purkinje cell layer.
Figure 5
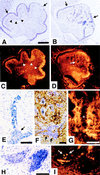
Abnormal neuronal cell migration in CXCR4−/− cerebellum. Parasagittal sections of E18.5 cerebellum with anterior to the left and dorsal to the top. (A and B) Nissl staining. The EGL (arrows) of wild-type cerebellum (A) lies between the meninges and the diffuse Purkinje cell layer (partially marked by arrowheads). The abnormal cerebellum in CXCR4−/− mice (B) has an irregular EGL (arrows) and large chromophilic cell clumps within the cerebellar anlage. (C and D) Calbindin immunohistochemistry. In comparison with the Purkinje cell layer (arrowheads) in wild-type cerebellum (C), Purkinje cells are located ectopically (arrow) in CXCR4−/− cerebellum (D). (E_–_I) Chromophilic cell clumps in CXCR4−/− cerebellar anlage. Streaming cells from the EGL (E). Combined Nissl and calbindin staining (F). Purkinje cell neurites do not extend into the clumps (asterisks) (G). Adjacent sections stained by Nissl technique (H) or BrdUrd incorporation (I). Scale bar is 200 μm (A_–_D) and 50 μm (E_–_H).
Similar articles
- Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nagasawa T, et al. Nature. 1996 Aug 15;382(6592):635-8. doi: 10.1038/382635a0. Nature. 1996. PMID: 8757135 - Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine.
Onai N, Zhang Yy, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Onai N, et al. Blood. 2000 Sep 15;96(6):2074-80. Blood. 2000. PMID: 10979950 - The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4.
D'Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, Baggiolini M, Moser B. D'Apuzzo M, et al. Eur J Immunol. 1997 Jul;27(7):1788-93. doi: 10.1002/eji.1830270729. Eur J Immunol. 1997. PMID: 9247593 - A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection.
Nagasawa T, Tachibana K, Kishimoto T. Nagasawa T, et al. Semin Immunol. 1998 Jun;10(3):179-85. doi: 10.1006/smim.1998.0128. Semin Immunol. 1998. PMID: 9653044 Review. - Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions.
Lapidot T. Lapidot T. Ann N Y Acad Sci. 2001 Jun;938:83-95. doi: 10.1111/j.1749-6632.2001.tb03577.x. Ann N Y Acad Sci. 2001. PMID: 11458529 Review.
Cited by
- Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells.
Guo R, Chai L, Chen L, Chen W, Ge L, Li X, Li H, Li S, Cao C. Guo R, et al. In Vitro Cell Dev Biol Anim. 2015 Jun;51(6):578-85. doi: 10.1007/s11626-014-9862-y. Epub 2015 Jan 31. In Vitro Cell Dev Biol Anim. 2015. PMID: 25636237 - Survival and Proliferation of Neural Progenitor-Derived Glioblastomas Under Hypoxic Stress is Controlled by a CXCL12/CXCR4 Autocrine-Positive Feedback Mechanism.
Calinescu AA, Yadav VN, Carballo E, Kadiyala P, Tran D, Zamler DB, Doherty R, Srikanth M, Lowenstein PR, Castro MG. Calinescu AA, et al. Clin Cancer Res. 2017 Mar 1;23(5):1250-1262. doi: 10.1158/1078-0432.CCR-15-2888. Epub 2016 Aug 19. Clin Cancer Res. 2017. PMID: 27542769 Free PMC article. - CXCL12: a new player in coronary disease identified through human genetics.
Farouk SS, Rader DJ, Reilly MP, Mehta NN. Farouk SS, et al. Trends Cardiovasc Med. 2010 Aug;20(6):204-9. doi: 10.1016/j.tcm.2011.08.002. Trends Cardiovasc Med. 2010. PMID: 22137643 Free PMC article. Review. - The chemokine X-factor: Structure-function analysis of the CXC motif at CXCR4 and ACKR3.
Wedemeyer MJ, Mahn SA, Getschman AE, Crawford KS, Peterson FC, Marchese A, McCorvy JD, Volkman BF. Wedemeyer MJ, et al. J Biol Chem. 2020 Oct 2;295(40):13927-13939. doi: 10.1074/jbc.RA120.014244. Epub 2020 Aug 11. J Biol Chem. 2020. PMID: 32788219 Free PMC article. - Cellular dynamics of neuronal migration in the hippocampus.
Hayashi K, Kubo K, Kitazawa A, Nakajima K. Hayashi K, et al. Front Neurosci. 2015 Apr 24;9:135. doi: 10.3389/fnins.2015.00135. eCollection 2015. Front Neurosci. 2015. PMID: 25964735 Free PMC article. Review.
References
- Luster A D. N Engl J Med. 1998;338:436–445. - PubMed
- Baggiolini M. Nature (London) 1998;392:565–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases