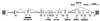Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme - PubMed (original) (raw)
Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme
K Ohno et al. Proc Natl Acad Sci U S A. 1998.
Abstract
In skeletal muscle, acetylcholinesterase (AChE) exists in homomeric globular forms of type T catalytic subunits (ACHET) and heteromeric asymmetric forms composed of 1, 2, or 3 tetrameric ACHET attached to a collagenic tail (ColQ). Asymmetric AChE is concentrated at the endplate (EP), where its collagenic tail anchors it into the basal lamina. The ACHET gene has been cloned in humans; COLQ cDNA has been cloned in Torpedo and rodents but not in humans. In a disabling congenital myasthenic syndrome, EP AChE deficiency (EAD), the normal asymmetric species of AChE are absent from muscle. EAD could stem from a defect that prevents binding of ColQ to ACHET or the insertion of ColQ into the basal lamina. In six EAD patients, we found no mutations in ACHET. We therefore cloned human COLQ cDNA, determined the genomic structure and chromosomal localization of COLQ, and then searched for mutations in this gene. We identified six recessive truncation mutations of COLQ in six patients. Coexpression of each COLQ mutant with wild-type ACHET in SV40-transformed monkey kidney fibroblast (COS) cells reveals that a mutation proximal to the ColQ attachment domain for ACHET prevents association of ColQ with ACHET; mutations distal to the attachment domain generate a mutant approximately 10.5S species of AChE composed of one ACHET tetramer and a truncated ColQ strand. The approximately 10.5S species lack part of the collagen domain and the entire C-terminal domain of ColQ, or they lack only the C-terminal domain, which is required for formation of the triple collagen helix, and this likely prevents their insertion into the basal lamina.
Figures
Figure 1
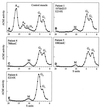
Density gradient centrifugation of AChE extracted from muscle in control subject (A) and EAD patients 1, 4, 5, and 6 (B_–_E). Mutations in each case are indicated. A and G denote asymmetric and globular species. In each patient extract, the heteromeric A12 and A8 species are absent and the composite G4/A4 peak is replaced by a single mutant ≈10.5S peak. (M, mutant peak.)
Figure 2
cDNA sequence of the human COLQ gene (GenBank accession no. AF057036) and truncation mutations in the patients. Mutations are underlined. The 107del215 mutation results from skipping of exons 2 and 3. Vertical lines indicate exon boundaries. The left and right columns show nucleotide and codon numbers from the translational start site. The 3′ untranslated region is not shown.
Figure 3
Alignment of the amino acid sequences of human, rat (14), partial mouse (14), and Torpedo (13) ColQ and truncation mutations in the patients. Gaps are inserted for alignment. The amino acid sequence encoded by the alternatively transcribed exon 1A also is shown. Closed arrowheads point to the first amino acid deleted by the truncation mutations. Dashes show identical amino acids. Secretion signal peptides are italicized for all species. Collagen domains consisting of GXY triplets are underlined. A black bar indicates the PRAD. Hatched bars indicate heparan sulfate proteoglycan binding domains. Open arrowheads show essential cysteines. The right column indicates codon numbers.
Figure 4
Genomic structure of the human COLQ gene. Exons (boxes) and introns (horizontal lines) are drawn to scale. Splicing marks indicate seven alternative transcripts found in normal controls. Hatched areas show untranslated regions. Closed arrowheads indicate point mutations; black bar indicates skipping of exons 2 and 3.
Figure 5
Chromosomal localization of COLQ by FISH. In situ hybridization was performed by using a digoxigenin-labeled human COLQ cDNA probe and normal metaphase cells from stimulated blood culture. (A) The digitized image of the COLQ probe signal is localized on both chromatids of 4′,6-diamidino-2-phenylindole-enhanced G-banded chromosome 3 from reverse imaging by the Vysis
smartcapture
system (Vysis, Downers Grove, IL). (B) COLQ is mapped to 3p25
Figure 6
Restriction analysis (A, B, D, and F) and allele-specific PCR (ASP) (C and E) using genomic DNA from patients and their respective relatives. (A to F) Families of patients 1 to 6, respectively. Closed and open arrowheads point to mutant and wild-type fragments. Closed symbols and arrows indicate patients; half-shaded symbols represent asymptomatic carriers. The −46G/A polymorphism in A is linked to the 107del215 mutation.
Figure 7
Sedimentation profiles of AChE species extracted from COS cells transfected with wild-type _ACHE_T and COLQ (A), wild-type _ACHE_T (B), and with wild-type _ACHE_T plus COLQ mutants (C_–_H). Solid lines in all panels indicate sedimentation in the presence of 0.5% Triton X-100; interrupted lines in A and E show sedimentation in the presence of 1% Brij-97. (M, mutant peak.)
Comment in
- Mutations causing muscle weakness.
Lindstrom J. Lindstrom J. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9070-1. doi: 10.1073/pnas.95.16.9070. Proc Natl Acad Sci U S A. 1998. PMID: 9689034 Free PMC article. No abstract available.
Similar articles
- The spectrum of mutations causing end-plate acetylcholinesterase deficiency.
Ohno K, Engel AG, Brengman JM, Shen XM, Heidenreich F, Vincent A, Milone M, Tan E, Demirci M, Walsh P, Nakano S, Akiguchi I. Ohno K, et al. Ann Neurol. 2000 Feb;47(2):162-70. Ann Neurol. 2000. PMID: 10665486 - Three novel COLQ mutations and variation of phenotypic expressivity due to G240X.
Shapira YA, Sadeh ME, Bergtraum MP, Tsujino A, Ohno K, Shen XM, Brengman J, Edwardson S, Matoth I, Engel AG. Shapira YA, et al. Neurology. 2002 Feb 26;58(4):603-9. doi: 10.1212/wnl.58.4.603. Neurology. 2002. PMID: 11865139 - The mammalian gene of acetylcholinesterase-associated collagen.
Krejci E, Thomine S, Boschetti N, Legay C, Sketelj J, Massoulié J. Krejci E, et al. J Biol Chem. 1997 Sep 5;272(36):22840-7. doi: 10.1074/jbc.272.36.22840. J Biol Chem. 1997. PMID: 9278446 - The origin of the molecular diversity and functional anchoring of cholinesterases.
Massoulié J. Massoulié J. Neurosignals. 2002 May-Jun;11(3):130-43. doi: 10.1159/000065054. Neurosignals. 2002. PMID: 12138250 Review. - Acetylcholinesterase: C-terminal domains, molecular forms and functional localization.
Massoulié J, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S. Massoulié J, et al. J Physiol Paris. 1998 Jun-Aug;92(3-4):183-90. doi: 10.1016/s0928-4257(98)80007-7. J Physiol Paris. 1998. PMID: 9789805 Review.
Cited by
- Current status of the congenital myasthenic syndromes.
Engel AG. Engel AG. Neuromuscul Disord. 2012 Feb;22(2):99-111. doi: 10.1016/j.nmd.2011.10.009. Epub 2011 Nov 21. Neuromuscul Disord. 2012. PMID: 22104196 Free PMC article. Review. - MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction.
Cartaud A, Strochlic L, Guerra M, Blanchard B, Lambergeon M, Krejci E, Cartaud J, Legay C. Cartaud A, et al. J Cell Biol. 2004 May 24;165(4):505-15. doi: 10.1083/jcb.200307164. J Cell Biol. 2004. PMID: 15159418 Free PMC article. - Cholinesterases in Tripartite Neuromuscular Synapse.
Petrov KA, Proskurina SE, Krejci E. Petrov KA, et al. Front Mol Neurosci. 2021 Dec 23;14:811220. doi: 10.3389/fnmol.2021.811220. eCollection 2021. Front Mol Neurosci. 2021. PMID: 35002624 Free PMC article. Review. - Congenital myasthenia-related AChR delta subunit mutation interferes with intersubunit communication essential for channel gating.
Shen XM, Fukuda T, Ohno K, Sine SM, Engel AG. Shen XM, et al. J Clin Invest. 2008 May;118(5):1867-76. doi: 10.1172/JCI34527. J Clin Invest. 2008. PMID: 18398509 Free PMC article. - Congenital myasthenic syndromes.
Finsterer J. Finsterer J. Orphanet J Rare Dis. 2019 Feb 26;14(1):57. doi: 10.1186/s13023-019-1025-5. Orphanet J Rare Dis. 2019. PMID: 30808424 Free PMC article.
References
- Maselli R A, Soliven B C. Muscle Nerve. 1991;14:1182–1188. - PubMed
- Salpeter M M, Kasprzak H, Feng H, Fertuck H. J Neurocytol. 1979;8:95–115. - PubMed
- Dettbarn W D. Fundam Appl Toxicol. 1984;4:S18–S26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases