Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp - PubMed (original) (raw)
Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp
H Inoue et al. Mol Biol Cell. 1998 Aug.
Free PMC article
Abstract
Decapentaplegic (Dpp) plays an essential role in Drosophila development, and analyses of the Dpp signaling pathway have contributed greatly to understanding of the actions of the TGF-beta superfamily. Intracellular signaling of the TGF-beta superfamily is mediated by Smad proteins, which are now grouped into three classes. Two Smads have been identified in Drosophila. Mothers against dpp (Mad) is a pathway-specific Smad, whereas Daughters against dpp (Dad) is an inhibitory Smad genetically shown to antagonize Dpp signaling. Here we report the identification of a common mediator Smad in Drosophila, which is closely related to human Smad4. Mad forms a heteromeric complex with Drosophila Smad4 (Medea) upon phosphorylation by Thick veins (Tkv), a type I receptor for Dpp. Dad stably associates with Tkv and thereby inhibits Tkv-induced Mad phosphorylation. Dad also blocks hetero-oligomerization and nuclear translocation of Mad. We also show that Mad exists as a monomer in the absence of Tkv stimulation. Tkv induces homo-oligomerization of Mad, and Dad inhibits this step. Finally, we propose a model for Dpp signaling by Drosophila Smad proteins.
Figures
Figure 1
Alignment of the predicted protein sequence of Drosophila Smad4/Medea with those of human Smad4, Mad, and Dad. The first methionine of Medea shown was chosen based on its similarity to that of human Smad4. The open reading frame encodes 745 amino acids. Dashes were inserted to maximize the alignment score. Residues identical to those of Medea are highlighted. The region located between triangles is the linker. Medea and Smad4 are highly conserved in the MH1 and MH2 regions. Note that Medea is longer than human Smad4 by ∼200 amino acids because of its long linker region. The Medea sequence has been deposited in GenBank with accession number AF057162.
Figure 2
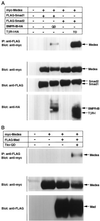
Interaction of Medea with pathway-specific Smads. (A) FLAG-tagged Smad1 or FLAG-tagged Smad2 was introduced into COS cells with myc-tagged Medea in the absence or presence of the HA-tagged constitutively active type I receptor for BMP (BMPR-IB; QD) or TGF-β (TβR-I; TD). Interaction was detected by immunoprecipitation with anti-FLAG antibody followed by Western blotting with anti-myc antibody. The expression of Medea, Smad1, Smad2 and receptors was monitored by straight Western blotting with antibodies against the respective tags. (B) Association of Mad with Medea was examined in a similar experiment. FLAG-tagged Mad and myc-tagged Medea were expressed in COS cells in the absence or presence of constitutively active Tkv (Tkv-QD). The expression of each protein was monitored by straight Western blotting.
Figure 3
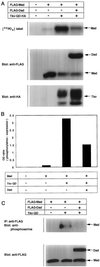
Phosphorylation of Mad by activated Tkv. (A) FLAG-tagged Mad was transfected into COS cells with or without Dpp receptors (Punt and Tkv). Cells were then labeled with [32P]orthophosphate, treated with 300 ng/ml BMP-2 for 1 h, lysed, and subjected to gel electrophoresis followed by analyses with a phosphorimager. The expression level of Mad was monitored by Western blotting. (B) Phosphorylation of Mad by Tkv was examined by Western blotting. Mad was immunoprecpitated with anti-FLAG antibody and immunoblotted with anti-phosphoserine antibody.
Figure 4
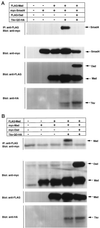
Inhibition by Dad of Tkv-induced phosphorylation of Mad. (A) Inhibition by Dad of Mad phosphorylation was examined by [32P]orthophosphate labeling. The experiment was performed as shown in Figure 3A. Expression levels of proteins were monitored by Western blotting. (B) Intensities of bands were determined with a densitometer, and the ratio between [32P]orthophosphate incorporation and protein expression level was calculated. (C) Effect of Dad on Tkv-induced Mad phosphorylation was examined. Anti-phosphoserine antibody was used as in Figure 3B. Expression levels of the proteins were monitored by Western blotting.
Figure 5
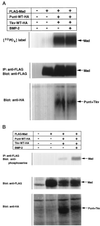
Interaction of Mad and Dad with Tkv. (A) Affinity cross-linking using 125I-BMP-2 was performed to examine the interaction of Mad and Dad with Tkv. Cells were transfected with FLAG-tagged Mad (lanes 1 and 2) or Dad (lanes 3 and 4) with combinations of wild-type (WT; lanes 1 and 3) or kinase-defective (KR; lanes 2 and 4) Tkv and Punt and affinity labeled with 125I-BMP-2. The lysates were subjected to immunoprecipitation with anti-FLAG antibody and detection by SDS-PAGE. Expression levels of proteins were monitored by Western blotting. (B) Interaction of FLAG-tagged Dad with HA-tagged Tkv was examined by immunoprecipitation with anti-FLAG antibody followed by Western blotting with anti-HA antibody. Wild-type (WT), the constitutively active form (QD), and the kinase-defective form (KR) of Tkv were used. Expression levels of proteins were monitored by Western blotting. (C) Effect of Dad expression on the interaction of Mad with Tkv was examined by affinity cross-linking followed by immunoprecipitation. Mad, Dad, and receptors were tagged with FLAG, myc, and HA, respectively. For coimmunoprecipitation, anti-FLAG antibody was used. Expression levels of proteins were monitored by Western blotting.
Figure 6
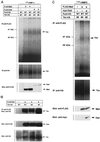
Inhibition of Tkv-induced oligomerization of Mad by Dad. (A) The effect of Dad on hetero-oligomerization of Mad with Smad4 was examined. Cells were transfected with the indicated combinations of plasmids. The lysates were subjected to immunoprecipitation with anti-FLAG antibody followed by immunoblotting with anti-myc antibody. Expression levels of proteins were monitored by Western blotting. (B) Effect of Dad on homo-oligomerization of Mad was examined. Cells were transfected with the indicated combinations of plasmids including FLAG-tagged and myc-tagged Mad. The lysates were subjected to immunoprecipitation with anti-FLAG antibody followed by immunoblotting with anti-myc antibody. Expression levels of proteins were monitored by Western blotting.
Figure 7
Inhibition of nuclear translocation of Mad by Dad. Nuclear translocation of Mad in the absence or presence of Dad was examined by immunostaining. COS cells were transfected with various combinations of Mad, Dad, and Tkv-QD. Anti-FLAG antibody was used as the first antibody. Subcellular localization was detected using FITC-labeled streptavidin, and fluorescence microscopy. (A) Mad; (B) Mad + Tkv-QD; (C) Mad + Tkv-QD + Dad. A representative cell, in each case, of higher magnification is shown in the inset.
Figure 8
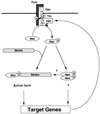
Model of the Dpp signaling pathway: cascades of phosphorylation and dynamic rearrangement of protein interactions. Dpp induces hetero-oligomerization of Punt and Tkv. Punt activates Tkv by transphosphorylating the juxtamembrane region. Activated Tkv phosphorylates Mad. Mad forms homo-oligomeric complexes and/or hetero-oligomeric complexes with Medea. It is not known whether Mad homo-oligomers have specific activity in vivo. Hetero-oligomers of Mad and Medea translocate into the nucleus where they transactivate target genes such as vestigial. Dad is one such target, and its expression is induced by Dpp. Dad stably binds to Tkv and interrupts phosphorylation of Mad by Tkv. The numbers of each Smad molecule in the homo- and hetero-oligomeric complexes remain to be determined.
Similar articles
- Medea is a Drosophila Smad4 homolog that is differentially required to potentiate DPP responses.
Wisotzkey RG, Mehra A, Sutherland DJ, Dobens LL, Liu X, Dohrmann C, Attisano L, Raftery LA. Wisotzkey RG, et al. Development. 1998 Apr;125(8):1433-45. doi: 10.1242/dev.125.8.1433. Development. 1998. PMID: 9502724 - The Drosophila gene Medea demonstrates the requirement for different classes of Smads in dpp signaling.
Das P, Maduzia LL, Wang H, Finelli AL, Cho SH, Smith MM, Padgett RW. Das P, et al. Development. 1998 Apr;125(8):1519-28. doi: 10.1242/dev.125.8.1519. Development. 1998. PMID: 9502733 - The Drosophila Medea gene is required downstream of dpp and encodes a functional homolog of human Smad4.
Hudson JB, Podos SD, Keith K, Simpson SL, Ferguson EL. Hudson JB, et al. Development. 1998 Apr;125(8):1407-20. doi: 10.1242/dev.125.8.1407. Development. 1998. PMID: 9502722 - TGF-beta family signal transduction in Drosophila development: from Mad to Smads.
Raftery LA, Sutherland DJ. Raftery LA, et al. Dev Biol. 1999 Jun 15;210(2):251-68. doi: 10.1006/dbio.1999.9282. Dev Biol. 1999. PMID: 10357889 Review. - Nuclear interpretation of Dpp signaling in Drosophila.
Affolter M, Marty T, Vigano MA, Jaźwińska A. Affolter M, et al. EMBO J. 2001 Jul 2;20(13):3298-305. doi: 10.1093/emboj/20.13.3298. EMBO J. 2001. PMID: 11432817 Free PMC article. Review.
Cited by
- Cross-talk between nitric oxide and transforming growth factor-beta1 in malaria.
Vodovotz Y, Zamora R, Lieber MJ, Luckhart S. Vodovotz Y, et al. Curr Mol Med. 2004 Nov;4(7):787-97. doi: 10.2174/1566524043359999. Curr Mol Med. 2004. PMID: 15579025 Free PMC article. Review. - Germline soma communication mediated by gap junction proteins regulates epithelial morphogenesis.
Sahu A, Karmakar S, Halder S, Ghosh G, Acharjee S, Dasgupta P, Ghosh R, Deshpande G, Prasad M. Sahu A, et al. PLoS Genet. 2021 Aug 3;17(8):e1009685. doi: 10.1371/journal.pgen.1009685. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34343194 Free PMC article. - Simple Expression Domains Are Regulated by Discrete CRMs During Drosophila Oogenesis.
Revaitis NT, Marmion RA, Farhat M, Ekiz V, Wang W, Yakoby N. Revaitis NT, et al. G3 (Bethesda). 2017 Aug 7;7(8):2705-2718. doi: 10.1534/g3.117.043810. G3 (Bethesda). 2017. PMID: 28634244 Free PMC article. - Regulation of TGF-β Family Signaling by Inhibitory Smads.
Miyazawa K, Miyazono K. Miyazawa K, et al. Cold Spring Harb Perspect Biol. 2017 Mar 1;9(3):a022095. doi: 10.1101/cshperspect.a022095. Cold Spring Harb Perspect Biol. 2017. PMID: 27920040 Free PMC article. Review. - Neuroligin 4 regulates synaptic growth via the bone morphogenetic protein (BMP) signaling pathway at the Drosophila neuromuscular junction.
Zhang X, Rui M, Gan G, Huang C, Yi J, Lv H, Xie W. Zhang X, et al. J Biol Chem. 2017 Nov 3;292(44):17991-18005. doi: 10.1074/jbc.M117.810242. Epub 2017 Sep 14. J Biol Chem. 2017. PMID: 28912273 Free PMC article.
References
- Abdollah S, Macías SM, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. - PubMed
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Brummel TJ, Twombly V, Marques G, Wrana JL, Newfeld SJ, Attisano L, Massagué J, O’Connor MB, Gelbart WM. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–261. - PubMed
- Chen X, Rubock MJ, Whitman M. A transcriptional partner for MAD proteins in TGF-β signalling. Nature. 1996;383:691–696. - PubMed
- Das P, Maduzia LL, Wang H, Finelli AL, Cho S-H, Smith MM, Padgett RW. The Drosophila gene Medea demonstrates the requirement for different classes of Smads in dpp signaling. Development. 1998;125:1519–1528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous