Gap junctional communication modulates gene expression in osteoblastic cells - PubMed (original) (raw)
Gap junctional communication modulates gene expression in osteoblastic cells
F Lecanda et al. Mol Biol Cell. 1998 Aug.
Free PMC article
Abstract
Bone-forming cells are organized in a multicellular network interconnected by gap junctions. In these cells, gap junctions are formed by connexin43 (Cx43) and connexin45 (Cx45). Cx43 gap junctions form pores that are more permeable to negatively charged dyes such as Lucifer yellow and calcein than are Cx45 pores. We studied whether altering gap junctional communication by manipulating the relative expression of Cx43 and Cx45 affects the osteoblast phenotype. Transfection of Cx45 in cells that express primarily Cx43 (ROS 17/2.8 and MC3T3-E1) decreased both dye transfer and expression of osteocalcin (OC) and bone sialoprotein (BSP), genes pivotal to bone matrix formation and calcification. Conversely, transfection of Cx43 into cells that express predominantly Cx45 (UMR 106-01) increased both cell coupling and expression of OC and BSP. Transient cotransfection of promoter-luciferase constructs and connexin expression vectors demonstrated that OC and BSP gene transcription was down-regulated by Cx45 cotransfection in ROS 17/2. 8 and MC3T3-E1 cells, in association with a decrease in dye coupling. Conversely, cotransfection of Cx43 in UMR 106-01 cells up-regulated OC and BSP gene transcription. Activity of other less specific osteoblast promoters, such as osteopontin and osteonectin, was less sensitive to changes in gap junctional communication. Thus, altering gap junctional permeability by manipulating the expression of Cx43 and Cx45 in osteoblastic cells alters transcriptional activity of osteoblast-specific promoters, presumably via modulation of signals that can diffuse from cell to cell. A communicating intercellular network is required for the full elaboration of a differentiated osteoblastic phenotype.
Figures
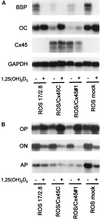
Figure 1
Steady-state mRNA levels of osteoblast products are altered by overexpression of chick Cx45 in ROS 17/2.8 osteoblastic cells. Cells stably expressing chick Cx45 (ROS/Cx45) or mock-transfected cells (ROS mock), as well as their parent cells (ROS 17/2.8), were grown to confluence in the same conditions, in the presence (+) or in the absence (−) of 10−8 M 1,25(OH)2D3, and poly-A RNA was separated on agarose gel and blotted onto nylon membranes. Membranes were sequentially hybridized with [32P]-labeled cDNA probes for (A) OC, BSP, Cx45, GAPDH, and (B) alkaline phosphatase (AP), ON, and OP. Between each hybridization step, the membrane was extensively washed and re-exposed to x-rays to ensure complete removal of the previous hybrizidation band.
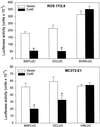
Figure 2
Transient transfection of chick Cx45 decreases transcriptional activity of OC and BSP promoters in ROS 17/2.8 (A) and MC3T3-E1 (B) osteoblastic cells. Cells were cotransfected with either a chick Cx45 expression construct or its vector, and one of the promoter-luciferase constructs, OCLUC, BSPLUC, SV40LUC, or ONLUC, as indicated. Luciferase activity was measured 3 d after transfection. Data are representative of four different experiments and are expressed as the average ± SD of triplicate wells for each condition. *, p < 0.01 vs. vector- transfected cells (t test for unpaired samples).
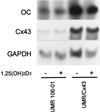
Figure 3
Steady-state OC mRNA levels are increased by overexpression of rat Cx43 in UMR 106–01 osteoblastic cells. UMR 106–01 cells stably expressing rat Cx43 (UMR/Cx43) and their parent cells were grown to confluence in the same culture conditions, and poly-A RNA was separated on agarose gel and blotted onto nylon membranes. Membranes were sequentially hybridized with [32P]-labeled cDNA probes for OC, Cx43, and GAPDH, with extensive washes between each hybridization step.
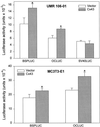
Figure 4
Transient transfection of rat Cx43 increases transcriptional activity of OC and BSP promoters in UMR 106/01 and MC3T3-E1 cells. Cells were cotransfected with either a rat Cx43 expression construct or its vector, and one of the promoter–luciferase constructs, OCLUC, BSPLUC or SV40LUC, as indicated. Luciferase activity was measured 3 d after transfection. Data are representative of four (UMR 106–01) and six (MC3T3-E1) different experiments and are expressed as the average ± SD of triplicate wells for each condition. *, p < 0.01 vs. vector-transfected cells (t test for unpaired samples).
Figure 5
Transient transfection of Cx45 in ROS 17/2.8 and Cx43 in UMR 106–01 cells leads to cell surface expression of either connexin protein in a proportion of cells. Cells were seeded at low density on glass coverslips and incubated with either Cx45 or Cx43 expression constructs for 72 h. After fixation, cells were immunostained with anti-Cx45 (ROS 17/2.8, left) or anti-Cx43 (UMR 106–01, right) antibody, as indicated. Bar, 20 μm.
Similar articles
- Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43.
Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. Koval M, et al. J Cell Biol. 1995 Aug;130(4):987-95. doi: 10.1083/jcb.130.4.987. J Cell Biol. 1995. PMID: 7642714 Free PMC article. - Inhibiting gap junctional intercellular communication alters expression of differentiation markers in osteoblastic cells.
Li Z, Zhou Z, Yellowley CE, Donahue HJ. Li Z, et al. Bone. 1999 Dec;25(6):661-6. doi: 10.1016/s8756-3282(99)00227-6. Bone. 1999. PMID: 10593410 - Modulation of connexin43 alters expression of osteoblastic differentiation markers.
Li Z, Zhou Z, Saunders MM, Donahue HJ. Li Z, et al. Am J Physiol Cell Physiol. 2006 Apr;290(4):C1248-55. doi: 10.1152/ajpcell.00428.2005. Epub 2005 Nov 30. Am J Physiol Cell Physiol. 2006. PMID: 16319124 - Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells.
Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, Wang HZ, Warlow PM, Westphale EM, Laing JG, et al. Steinberg TH, et al. EMBO J. 1994 Feb 15;13(4):744-50. doi: 10.1002/j.1460-2075.1994.tb06316.x. EMBO J. 1994. PMID: 8112289 Free PMC article. - Temporal expression of neuronal connexins during hippocampal ontogeny.
Rozental R, Srinivas M, Gökhan S, Urban M, Dermietzel R, Kessler JA, Spray DC, Mehler MF. Rozental R, et al. Brain Res Brain Res Rev. 2000 Apr;32(1):57-71. doi: 10.1016/s0165-0173(99)00096-x. Brain Res Brain Res Rev. 2000. PMID: 10751657 Review.
Cited by
- ERK acts in parallel to PKCδ to mediate the connexin43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts.
Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. Niger C, et al. Am J Physiol Cell Physiol. 2012 Apr 1;302(7):C1035-44. doi: 10.1152/ajpcell.00262.2011. Epub 2012 Jan 25. Am J Physiol Cell Physiol. 2012. PMID: 22277757 Free PMC article. - Endothelin1-induced Ca(2+) mobilization is altered in calvarial osteoblastic cells of Cx43(+/- ) mice.
Geneau G, Defamie N, Mesnil M, Cronier L. Geneau G, et al. J Membr Biol. 2007 Jun;217(1-3):71-81. doi: 10.1007/s00232-007-9024-1. Epub 2007 Jun 14. J Membr Biol. 2007. PMID: 17568972 - Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress.
Jiang JX, Siller-Jackson AJ, Burra S. Jiang JX, et al. Front Biosci. 2007 Jan 1;12:1450-62. doi: 10.2741/2159. Front Biosci. 2007. PMID: 17127393 Free PMC article. Review. - Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation.
Mbalaviele G, Sheikh S, Stains JP, Salazar VS, Cheng SL, Chen D, Civitelli R. Mbalaviele G, et al. J Cell Biochem. 2005 Feb 1;94(2):403-18. doi: 10.1002/jcb.20253. J Cell Biochem. 2005. PMID: 15526274 Free PMC article. - Interleukin-1beta increases gap junctional communication among synovial fibroblasts via the extracellular-signal-regulated kinase pathway.
Niger C, Howell FD, Stains JP. Niger C, et al. Biol Cell. 2009 Oct 12;102(1):37-49. doi: 10.1042/BC20090056. Biol Cell. 2009. PMID: 19656083 Free PMC article.
References
- Beyer EC, Paul DL, Goodenough DA. Connexin family of the gap junction proteins. J Membr Biol. 1990;116:187–194. - PubMed
- Beyer EC, Steinberg TH. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J Biol Chem. 1991;266:7971–7974. - PubMed
- Boudreaux JM, Towler DA. Synergistic induction of osteocalcin gene expression. J Biol Chem. 1996;271:7508–7515. - PubMed
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
- Brown MEA, Arlot ME, Reeve J. Osteoblast density and the evolution of BMUs in vertebral osteoporosis. Bone. 1993;14:473–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous