Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A - PubMed (original) (raw)
Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A
M L Beckman et al. J Neurosci. 1998.
Abstract
Syntaxin 1A inhibits GABA uptake of an endogenous GABA transporter in neuronal cultures from rat hippocampus and in reconstitution systems expressing the cloned rat brain GABA transporter GAT1. Evidence of interactions between syntaxin 1A and GAT1 comes from three experimental approaches: botulinum toxin cleavage of syntaxin 1A, syntaxin 1A antisense treatments, and coimmunoprecipitation of a complex containing GAT1 and syntaxin 1A. Protein kinase C (PKC), shown previously to modulate GABA transporter function, exerts its modulatory effects by regulating the availability of syntaxin 1A to interact with the transporter, and a transporter mutant that fails to interact with syntaxin 1A is not regulated by PKC. These results suggest a new target for regulation by syntaxin 1A and a novel mechanism for controlling the machinery involved in both neurotransmitter release and reuptake.
Figures
Fig. 1.
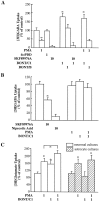
GABA uptake in hippocampal neurons is regulated by botulinum toxin C1 and protein kinase C. A, Modulation of GABA uptake in neuronal cultures. Drug and toxin concentrations (in μ
m
) are shown below the graph. Pretreatment (30 min) of cultures with PMA, but not 4αPDD, decreased [3H]GABA uptake. Uptake was blocked in all conditions tested by the GAT1 antagonist SKF89976A. Pretreatment (30 min) of cultures with BONT/C1, but not BONT/B, increased [3H]GABA uptake and prevented the PMA-mediated decrease. Data are from four separate experiments, three wells per condition per experiment. GABA uptake under control conditions ranged from 716 to 1211 fmol/min per mg of protein. B, Modulation of GABA uptake in astrocyte cultures. Astrocyte GABA uptake is not modulated by PKC or botulinum toxin. Treatments are described in_A_. Data are from three experiments, at least four wells per condition per experiment. GABA uptake under control conditions ranged from 116 to 323 fmol/min per mg of protein.C, Modulation of glutamate uptake in neuronal and astrocyte cultures. Treatments are described in A. Data are from three experiments, eight wells per condition per experiment. Mean neuronal glutamate uptake under control conditions was 897 fmol/min per mg of protein; mean astrocyte glutamateuptake under control conditions was 2714 fmol/min per mg of protein. Experimental conditions that resulted in a significant change (p < 0.05) from control values or between the indicated groups are denoted by an_asterisk_.
Fig. 2.
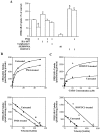
PC12 cells expressing GAT1 are regulated by botulinum toxin C1 and protein kinase C. A, GAT1 regulation in PC12 cells mimics endogenous GABA transporter regulation. Cells were treated as described in Figure 1_A_ and the Results. Data are from two to six different experiments, at least three wells per condition per experiment. GABA uptake under control conditions ranged from 2744 to 8297 fmol/min per mg of protein. Experimental conditions that resulted in a significant change (p < 0.05) from control values are denoted by an asterisk. B, PMA decreases transport Vmax (open circles) compared with that in untreated controls (filled circles).Top, Saturation analysis was performed at six different GABA concentrations for cells in the presence or absence of 1 μ
m
PMA. Bottom, Eadie-Hofstee transformations of these data are shown. C, BONT/C1 increases transport Vmax (open circles) compared with that in untreated cells (filled circles). Experiments are described in_B_. BONT/C1 concentration was 1 μ
m
. Saturation experiments were performed twice; data shown are from one experiment.
Fig. 3.
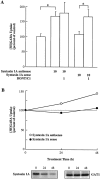
Inhibiting syntaxin 1A expression upregulates GAT1 transport in PC12 cells. A, Syntaxin 1A antisense oligonucleotide treatment of PC12 cells causes an increase in GABA uptake. PC12 cells expressing GAT1 were incubated for 48 hr with 10 μ
m
antisense or sense oligonucleotides as described in Materials and Methods. Some cells were treated 30 min before assay with 1 μ
m
BONT/C1. Data are from three experiments, six wells per condition per experiment. GABA uptake under control conditions ranged from 1865 to 2767 fmol/min per mg of protein. Experimental conditions that resulted in a significant difference (p < 0.05) between the two identified groups are denoted by an asterisk. B, The increase in GABA uptake correlates with a decrease in syntaxin 1A expression. PC12 cells were treated as described in A. [3H]GABA uptake experiments were performed 0, 24, or 48 hr after oligonucleotide application. Parallel samples were harvested for Western blot analysis and probed with antibodies to either syntaxin 1A (lower left blot) or GAT1 (lower right blot).
Fig. 4.
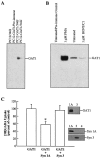
Syntaxin 1A interacts with GAT1. A, Syntaxin 1A and GAT1 coimmunoprecipitate. PC12 cells expressing GAT1 were precipitated with anti-syntaxin 1A antibody, and GAT1 was visualized by Western blot using two different anti-GAT1 antibodies (346J and 342J). Wild-type PC12 cells probed with antibody 346J and PC12-GAT1 cells probed with preimmune serum served as controls. Antibody 346J was used in all further immunoblot experiments.B, Protein kinase C regulates the interaction between syntaxin 1A and GAT1. Cells were pretreated for 30 min with PMA or BONT/C1 before precipitation with the syntaxin 1A antibody.C, The interaction between syntaxin 1A and GAT1 is specific. 1F9 cells were cotransfected with either syntaxin 1A (Syn 1A) or syntaxin 3 (Syn 3). Forty-eight hours later, [3H]GABA uptake assays were performed. Data are from three experiments, six wells per condition per experiment. GABA uptake under control conditions ranged from 365 to 923 fmol/min per mg of protein. Experimental conditions that resulted in a significant change (p < 0.05) from control values are denoted by an asterisk.Top blot, Immunoprecipitation with either syntaxin 1A or syntaxin 3 antibodies was performed on parallel samples and Western blotted with the GAT1 antibody. Bottom blots, Specificity of each antibody for syntaxin 1A and syntaxin 3 was determined by immunoblot of membranes prepared from oocytes expressing either syntaxin subtype. For all immunoblot experiments, equal amounts of protein were added in each lane.
Fig. 5.
GAT1 mutants that fail to interact with syntaxin 1A are not regulated by PKC. A, A GAT1 mutant lacking a leucine heptad repeat sequence does not interact with syntaxin 1A. Mutant GABA transporters lacking the cytoplasmic N-terminal tail (ΔN) or a leucine heptad repeat (Δ4L) were expressed in PC12 cells. [3H]GABA uptake assays were performed on cultures treated with PMA and were compared with untreated cultures. Only the control result for the wild-type (WT) condition is shown. The mean control GABA uptake values for each condition (in fmol/min per mg of protein) were as follows: wild-type, 1337;ΔN, 2697; and Δ4L, 1657. In parallel cultures, the Δ4L mutant could not be immunoprecipitated by a syntaxin 1A antibody (blot).B, The GAT1Δ4L mutant is insensitive to regulation by PKC or BONT/C1. Data are from two experiments, six wells per condition per experiment. The mean control GABA uptake values for each condition (in fmol/min per mg of protein) were as follows: wild-type, 1115; and_Δ4L_, 1712. Experimental conditions that resulted in a significant change (p < 0.05) from control values are denoted by an asterisk.
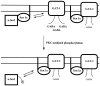
Fig. 6.
A model for the regulation of GAT1 by syntaxin 1A and protein kinase C. In the absence of PKC, syntaxin 1A can bind to many partners including GAT1. Phosphorylation of these binding partners, e.g., Munc18 or n-Sec1, results in a greater number of syntaxin 1A and GAT1 interactions.
Similar articles
- Syntaxin 1A inhibits GABA flux, efflux, and exchange mediated by the rat brain GABA transporter GAT1.
Wang D, Deken SL, Whitworth TL, Quick MW. Wang D, et al. Mol Pharmacol. 2003 Oct;64(4):905-13. doi: 10.1124/mol.64.4.905. Mol Pharmacol. 2003. PMID: 14500747 - Syntaxin 1A up-regulates GABA transporter expression by subcellular redistribution.
Horton N, Quick MW. Horton N, et al. Mol Membr Biol. 2001 Jan-Mar;18(1):39-44. Mol Membr Biol. 2001. PMID: 11396610 - Substrates regulate gamma-aminobutyric acid transporters in a syntaxin 1A-dependent manner.
Quick MW. Quick MW. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5686-91. doi: 10.1073/pnas.082712899. Proc Natl Acad Sci U S A. 2002. PMID: 11960023 Free PMC article. - SNARE-ing neurotransmitter transporters.
Blakely RD, Sung U. Blakely RD, et al. Nat Neurosci. 2000 Oct;3(10):969-71. doi: 10.1038/79898. Nat Neurosci. 2000. PMID: 11017164 Review. No abstract available. - Sorting of GABA transporter proteins in polarized cells.
Caplan MJ. Caplan MJ. Neurochem Int. 1996 Oct;29(4):357-60. doi: 10.1016/0197-0186(95)00159-x. Neurochem Int. 1996. PMID: 8939443 Review. No abstract available.
Cited by
- A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity.
Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. Sung U, et al. J Neurosci. 2003 Mar 1;23(5):1697-709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. J Neurosci. 2003. PMID: 12629174 Free PMC article. - The Caenorhabditis elegans snf-11 gene encodes a sodium-dependent GABA transporter required for clearance of synaptic GABA.
Mullen GP, Mathews EA, Saxena P, Fields SD, McManus JR, Moulder G, Barstead RJ, Quick MW, Rand JB. Mullen GP, et al. Mol Biol Cell. 2006 Jul;17(7):3021-30. doi: 10.1091/mbc.e06-02-0155. Epub 2006 Apr 26. Mol Biol Cell. 2006. PMID: 16641366 Free PMC article. - Pretreatment of rat brain synaptosomes with GABA increases subsequent GABA uptake via GABA(B) receptor activation.
Cupello A, Scarrone S. Cupello A, et al. Neurochem Res. 2001 Jan;26(1):11-4. doi: 10.1023/a:1007616229260. Neurochem Res. 2001. PMID: 11358276 - Membrane trafficking regulates the activity of the human dopamine transporter.
Melikian HE, Buckley KM. Melikian HE, et al. J Neurosci. 1999 Sep 15;19(18):7699-710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. J Neurosci. 1999. PMID: 10479674 Free PMC article. - Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders.
Malenfant P, Liu X, Hudson ML, Qiao Y, Hrynchak M, Riendeau N, Hildebrand MJ, Cohen IL, Chudley AE, Forster-Gibson C, Mickelson EC, Rajcan-Separovic E, Lewis ME, Holden JJ. Malenfant P, et al. J Autism Dev Disord. 2012 Jul;42(7):1459-69. doi: 10.1007/s10803-011-1389-4. J Autism Dev Disord. 2012. PMID: 22048961
References
- Asano T, Takata K, Katagiri H, Tsukuda K, Lin J-L, Ishihara H, Inukai K, Hirano H, Yazaki Y, Oka Y. Domains responsible for the differential targeting of glucose transporter isoforms. J Biol Chem. 1992;267:19636–19641. - PubMed
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
- Beckman ML, Quick MW (1998) Neurotransmitter transporters: regulators of function and functional regulation. J Membr Biol, in press. - PubMed
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources