Survival of cultured neurons from amyloid precursor protein knock-out mice against Alzheimer's amyloid-beta toxicity and oxidative stress - PubMed (original) (raw)
Survival of cultured neurons from amyloid precursor protein knock-out mice against Alzheimer's amyloid-beta toxicity and oxidative stress
A R White et al. J Neurosci. 1998.
Abstract
Studies on the amyloid precursor protein (APP) have suggested that it may be neuroprotective against amyloid-beta (Abeta) toxicity and oxidative stress. However, these findings have been obtained from either transfection of cell lines and mice that overexpress human APP isoforms or pretreatment of APP-expressing primary neurons with exogenous soluble APP. The neuroprotective role of endogenously expressed APP in neurons exposed to Abeta or oxidative stress has not been determined. This was investigated using primary cortical and cerebellar neuronal cultures established from APP knock-out (APP-/-) and wild-type (APP+/+) mice. Differences in susceptibility to Abeta toxicity or oxidative stress were not found between APP-/- and APP+/+ neurons. This observation may reflect the expression of the amyloid precursor-like proteins 1 and 2 (APLP1 and APLP2) molecules and supports the theory that APP and the APLPs may have similar functional activities. Increased expression of cell-associated APLP2, but not APLP1, was detected in Abeta-treated APP-/- and APP+/+ cultures but not in H2O2-treated cultures. This suggests that the Abeta toxicity pathway differs from other general forms of oxidative stress. These findings show that Abeta toxicity does not require an interaction of the Abeta peptide with the parental molecule (APP) and is therefore distinct from prion protein neurotoxicity that is dependent on the expression of the parental cellular prion protein.
Figures
Fig. 1.
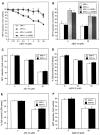
APP−/− and APP+/+ cortical neurons do not have differences in susceptibility to Aβ25–35 or Aβ1–42 inhibition of MTT reduction. Primary cortical neurons were grown at (A) high density (450,000 cells/cm2) or (B) low density (250,000 cells/cm2) for 3 d and exposed to Aβ25–35 for an additional 4 d. No differences between MTT reduction were observed between APP−/− and APP+/+ cortical neurons exposed to Aβ25–35 at either density. Treatment of cultures with 10 ng/ml bFGF (applied concomitantly with Aβ) resulted in a significant increase in cell viability as compared with non-bFGF-treated cultures when measured 4 d after exposure to Aβ25–35. *p < 0.05, **p < 0.01: differences in MTT reduction between bFGF and non-bFGF-treated cultures were determined using ANOVA and Newman–Keuls tests. C, APP−/− and APP+/+ cortical neurons reveal no differences in MTT reduction when treated with Aβ1–42. D, APP−/− and APP+/+ cortical neurons have no differences in susceptibility to Aβ25–35-induced cell death as determined using the LDH assay. E, APP−/− and APP+/+ cortical neurons reveal no differences in survival when exposed to Aβ1–42-induced cell death as determined using the LDH assay.F, APP−/− and APP+/+ cerebellar granule neurons reveal no differences in susceptibility to Aβ25–35 inhibition of MTT reduction. Primary cerebellar neurons were grown for 1 d and exposed to Aβ25–35 for an additional 6 d.
Fig. 2.
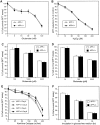
APP−/− and APP+/+ neurons do not have differences in susceptibility to intracellular- or extracellular-generated oxidative stress. A, Primary cortical neurons were grown for 2 d and exposed to glutamate for 24 hr. B, Primary cortical cultures were grown for 4 d and then exposed to H2O2 for 24 hr. C, Primary cerebellar granule neurons were grown for 7 d and then exposed to glutamate for 30 min. D, Primary cortical cultures were grown for 14 d and then exposed to glutamate for 30 min. Cell viability was determined 24 hr later. E, Primary cortical neurons were exposed to increasing concentrations of xanthine oxidase and 50 μ
m
xanthine for 24 hr at either 4 or 6 d in vitro. F, Primary cortical neurons were grown for 14 d before incubation in glucose-free Locke’s media. Cell viability was determined after the given incubation period.
Fig. 3.
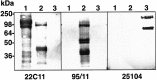
Characterization of the specificity of the APLP2 and APLP1 antibodies. Western blots of recombinant sAPP751 (lane 1), sAPLP2 (lane 2), and sAPLP1 (lane 3) probed with 22C11 (anti-APP/APLP2, 1:2000), 95/11 (anti-APLP2, 1:1000), or 25104 (anti-APLP1, 1:1000). The lower bands correspond to breakdown products as described previously (Henry et al., 1997). The position of the molecular weight markers is indicated on the left.
Fig. 4.
Quantitative immunoblotting of cell-associated APP, APLP1, and APLP2 in neurons exposed to Aβ25–35 and H2O2. Primary cortical neurons were grown for 2 d and then exposed to either 10 μ
m
Aβ25–35(Aβ) or 25 μ
m
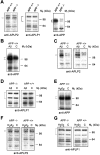
H2O2, or were untreated [control (C)] for 24 hr. The antibodies are anti-APP/APLP2 (22C11, 1:2000), anti-APLP2 (95/11, 1:1000), or anti-APLP1 (25104, 1:1000). The brackets correspond to the proteins described in Results and their molecular weights are as follows: anti-APP (95–105), anti-APLP1 (87 and 126), and anti-APLP2 (110 kDa). The position of the molecular weight markers is indicated on the_right-hand side_. A, Analysis of APLP2, APLP1, and APP expression under basal conditions. B, Analysis of APP expression detected in Aβ25–35-treated APP+/+ cultures. C, Analysis of APLP2 expression in Aβ25–35-treated APP−/− and APP+/+ neurons shows a significant increase in APLP2 expression in both APP−/− and APP+/+ neurons exposed to Aβ. D, Analysis of APLP1 expression in Aβ25–35-treated APP−/− or APP+/+ neurons.E, Analysis of APP expression in APP+/+ neurons in response to H2O2. F, Analysis of APLP2 expression in H2O2-treated APP−/− and APP+/+ neurons.G, Analysis of APLP1 expression in H2O2-treated APP−/− and APP+/+ neurons.
Similar articles
- Diverse fibrillar peptides directly bind the Alzheimer's amyloid precursor protein and amyloid precursor-like protein 2 resulting in cellular accumulation.
White AR, Maher F, Brazier MW, Jobling MF, Thyer J, Stewart LR, Thompson A, Gibson R, Masters CL, Multhaup G, Beyreuther K, Barrow CJ, Collins SJ, Cappai R. White AR, et al. Brain Res. 2003 Mar 21;966(2):231-44. doi: 10.1016/s0006-8993(02)04173-2. Brain Res. 2003. PMID: 12618346 - The Alzheimer's disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures.
White AR, Multhaup G, Maher F, Bellingham S, Camakaris J, Zheng H, Bush AI, Beyreuther K, Masters CL, Cappai R. White AR, et al. J Neurosci. 1999 Nov 1;19(21):9170-9. doi: 10.1523/JNEUROSCI.19-21-09170.1999. J Neurosci. 1999. PMID: 10531420 Free PMC article. - Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review.
Butterfield DA. Butterfield DA. Free Radic Res. 2002 Dec;36(12):1307-13. doi: 10.1080/1071576021000049890. Free Radic Res. 2002. PMID: 12607822 Review. - Processing of amyloid precursor protein and amyloid peptide neurotoxicity.
Nathalie P, Jean-Noël O. Nathalie P, et al. Curr Alzheimer Res. 2008 Apr;5(2):92-9. doi: 10.2174/156720508783954721. Curr Alzheimer Res. 2008. PMID: 18393795 Review.
Cited by
- Copper transport and Alzheimer's disease.
Macreadie IG. Macreadie IG. Eur Biophys J. 2008 Mar;37(3):295-300. doi: 10.1007/s00249-007-0235-2. Epub 2007 Nov 15. Eur Biophys J. 2008. PMID: 18004558 Review. - New insights into the role of fibroblast growth factors in Alzheimer's disease.
Alam R, Mrad Y, Hammoud H, Saker Z, Fares Y, Estephan E, Bahmad HF, Harati H, Nabha S. Alam R, et al. Mol Biol Rep. 2022 Feb;49(2):1413-1427. doi: 10.1007/s11033-021-06890-0. Epub 2021 Nov 3. Mol Biol Rep. 2022. PMID: 34731369 Review. - Sex-dependent effects of amyloid precursor-like protein 2 in the SOD1-G37R transgenic mouse model of MND.
Truong PH, Crouch PJ, Hilton JBW, McLean CA, Cappai R, Ciccotosto GD. Truong PH, et al. Cell Mol Life Sci. 2021 Oct;78(19-20):6605-6630. doi: 10.1007/s00018-021-03924-5. Epub 2021 Sep 2. Cell Mol Life Sci. 2021. PMID: 34476545 Free PMC article. - β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin.
Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI, Duce JA. Wong BX, et al. PLoS One. 2014 Dec 2;9(12):e114174. doi: 10.1371/journal.pone.0114174. eCollection 2014. PLoS One. 2014. PMID: 25464026 Free PMC article. - Oxidative stress, cell cycle, and neurodegeneration.
Klein JA, Ackerman SL. Klein JA, et al. J Clin Invest. 2003 Mar;111(6):785-93. doi: 10.1172/JCI18182. J Clin Invest. 2003. PMID: 12639981 Free PMC article. Review. No abstract available.
References
- Abe K, Kimura H. Amyloid β toxicity consists of a Ca2+-independent early phase and a Ca2+-dependent late phase. J Neurochem. 1996;67:2074–2078. - PubMed
- Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP. Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of β-amyloid precursor. J Neurochem. 1995;64:2087–2096. - PubMed
- Brown DR, Schmidt B, Kretzschmar HA. A neurotoxic prion protein fragment enhances proliferation of microglia but not astrocytes in culture. Glia. 1996a;18:59–67. - PubMed
- Brown DR, Schmidt B, Kretzschmar HA. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996b;380:345–347. - PubMed
- Brunden KR, Richter-Cook NJ, Chaturvedi N, Frederickson RCA. pH-dependent binding of synthetic β-amyloid peptides to glycosaminoglycans. J Neurochem. 1993;61:2147–2154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous