Specific differentiation between Mycobacterium bovis BCG and virulent strains of the Mycobacterium tuberculosis complex - PubMed (original) (raw)
Specific differentiation between Mycobacterium bovis BCG and virulent strains of the Mycobacterium tuberculosis complex
J Magdalena et al. J Clin Microbiol. 1998 Sep.
Abstract
A PCR procedure based on the intergenic region (IR) separating two genes encoding a recently identified mycobacterial two-component system, named SenX3-RegX3, was developed and was shown to be suitable for identifying Mycobacterium bovis BCG. The senX3-regX3 IR contains a novel type of repetitive sequence, called mycobacterial interspersed repetitive units (MIRUs). All tested BCG strains exclusively contained 77-bp MIRUs within the senX3-regX3 IR, whereas all non-BCG M. tuberculosis complex strains contained a 53-bp MIRU, in addition to the 77-bp MIRUs. All 148 strains analyzed so far could be divided into eight different groups according to the copy numbers of the 77-bp MIRU and to the presence or absence of the 53-bp MIRU. BCG strains contained either one, two, or three 77-bp MIRUs. The other strains contained one to five 77-bp MIRUs invariably followed by a 53-bp MIRU. The consistent absence of the 53-bp MIRU in BCG strains and its presence in virulent strains allowed us to develop an enzyme-linked immunosorbent assay using specific capture oligonucleotide probes to distinguish between BCG and other M. tuberculosis complex strains.
Figures
FIG. 1
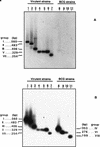
Agarose gel electrophoresis and Southern blot analysis of senX3-regX3 IR PCR products. The PCR products were analyzed by electrophoresis and ethidium bromide staining with a 2.5% agarose gel (A) and by Southern blot hybridization with a DIG-labeled 221-bp DNA fragment of M. tuberculosis 2296207 containing the senX3-regX3 IR as a probe (B). The senX3-regX3 IR PCR products were obtained from the Japanese (lanes 1), Russian (lanes 2), Glaxo (lanes 3), Montreal (lanes 4), and Prague (lanes 5) M. bovis BCG strains and from non-BCG M. bovis strains 60 (lanes 6), 63 (lanes 7), 78 (lanes 8), AN5 (lanes 9), 76 (lanes 10), and 1 (lanes 11). Positive (pRegX3Mt1 [lanes 12] and pRegX3Bc1 [lanes 13]) and negative (lanes 14) controls are also shown, as are molecular size markers (1-kb ladder; left and right lanes). The sizes of the PCR products that were obtained are indicated in the left and right margins.
FIG. 2
Schematic representation of the mycobacterial senX3-regX3 IR. (A) The 3.2-kb _Eco_RI-_Bam_HI fragment containing the senX3-regX3 operon is shown by the thin line. The senX3 and regX3 genes are indicated by the thicker arrows. The IRs of the different strains can be divided into eight groups. Five groups (groups I, II, III, V, and VII) correspond to virulent, non-BCG strains and contain variable copies of the 77-bp MIRU (thick arrows) and one copy of the 53-bp MIRU (thin arrow). Three groups (groups IV, VI, and VIII) correspond to avirulent, BCG strains and contain exclusively one to three 77-bp MIRUs. (B) The nucleotide and amino acid sequences of the 77- and 53-bp MIRUs are indicated by the single-letter code. The sequence differences between the 77- and 53-bp MIRUs are circled. The O77 oligonucleotide specific for the 77-bp MIRU and O53 specific for the 53-bp MIRU are boxed.
FIG. 3
Southern blot analysis of the senX3-regX3 IR PCR products. DIG-labeled O53 (A) and O77 (B) were used as probes for Southern blot analysis of the senX3-regX3 IR PCR products obtained from M. tuberculosis V.808 (lane 1), V.729 (lanes 2), and 2296207 (lane 6); from non-BCG M. bovis AN5 (lane 3), 76 (lane 4), 88367 (lane 5), and 1 (lane 7); and from the Japanese (lane 8), 1173P2 (lane 9), Glaxo (lane 10), and Prague (lane 11) BCG strains. The group numbers and sizes of the amplified DNA fragments are given in the left and right margins.
FIG. 4
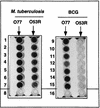
ELISA of the senX3-regX3 IR PCR products. Biotinylated oligonucleotides O77 and O53R were immobilized on streptavidin-coated microtiter plates and incubated with 10 μl of the DIG-labeled senX3-regX3 IR PCR products obtained from M. tuberculosis strains 1033 (wells 1), 1035 (wells 2), 1036 (wells 3), 1037 (wells 4), 1038 (wells 5), 1039 (wells 6), and 2296207 (wells 7) and the Japanese (wells 9), 1173P2 (wells 10), Glaxo (wells 11), Russian (wells 12), Danish (wells 13), Prague (wells 14) and Montreal (wells 15) BCG strains. Wells 8 contained no PCR product, and wells 16 contained neither the PCR product nor the oligonucleotides.
FIG. 5
Distribution of the various M. tuberculosis complex strains among eight different groups. The MIRU copy numbers of a total of 148 strains of the M. tuberculosis complex were determined in this study and in a previous study (19). The distributions of the strains are indicated as percentages for each group. Groups IV, VI, and VIII (black bars) contain only BCG strains. Groups I, II, III, V, and VII (white bars) contain only non-BCG strains.
Similar articles
- Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex.
Magdalena J, Vachée A, Supply P, Locht C. Magdalena J, et al. J Clin Microbiol. 1998 Apr;36(4):937-43. doi: 10.1128/JCM.36.4.937-943.1998. J Clin Microbiol. 1998. PMID: 9542912 Free PMC article. - Differentiation of strains in Mycobacterium tuberculosis complex by DNA sequence polymorphisms, including rapid identification of M. bovis BCG.
Frothingham R. Frothingham R. J Clin Microbiol. 1995 Apr;33(4):840-4. doi: 10.1128/jcm.33.4.840-844.1995. J Clin Microbiol. 1995. PMID: 7790448 Free PMC article. - Identification of novel intergenic repetitive units in a mycobacterial two-component system operon.
Supply P, Magdalena J, Himpens S, Locht C. Supply P, et al. Mol Microbiol. 1997 Dec;26(5):991-1003. doi: 10.1046/j.1365-2958.1997.6361999.x. Mol Microbiol. 1997. PMID: 9426136 - [Efficacy and safety of vaccines against tuberculosis in the relation to genetic variability of Mycobacterium bovis BCG strains].
Prygiel M, Janaszek-Seydlitz W, Bucholc B. Prygiel M, et al. Przegl Epidemiol. 2011;65(4):621-8. Przegl Epidemiol. 2011. PMID: 22390050 Review. Polish. - Microevolution of BCG substrains.
Krysztopa-Grzybowska K, Lutyńska A. Krysztopa-Grzybowska K, et al. Postepy Hig Med Dosw (Online). 2016 Dec 21;70(0):1259-1266. Postepy Hig Med Dosw (Online). 2016. PMID: 28026828 Review.
Cited by
- Mycobacterium microti tuberculosis in its maintenance host, the field vole (Microtus agrestis): characterization of the disease and possible routes of transmission.
Kipar A, Burthe SJ, Hetzel U, Rokia MA, Telfer S, Lambin X, Birtles RJ, Begon M, Bennett M. Kipar A, et al. Vet Pathol. 2014 Sep;51(5):903-14. doi: 10.1177/0300985813513040. Epub 2013 Dec 13. Vet Pathol. 2014. PMID: 24334995 Free PMC article. - Genotyping Mycobacterium bovis from cattle in the Central Pampas of Argentina: temporal and regional trends.
Shimizu E, Macías A, Paolicchi F, Magnano G, Zapata L, Fernández A, Canal A, Garbaccio S, Cataldi A, Caimi K, Zumárraga M. Shimizu E, et al. Mem Inst Oswaldo Cruz. 2014 Apr;109(2):236-45. doi: 10.1590/0074-0276140292. Epub 2014 Mar 4. Mem Inst Oswaldo Cruz. 2014. PMID: 24676658 Free PMC article. - An essential two-component signal transduction system in Mycobacterium tuberculosis.
Zahrt TC, Deretic V. Zahrt TC, et al. J Bacteriol. 2000 Jul;182(13):3832-8. doi: 10.1128/JB.182.13.3832-3838.2000. J Bacteriol. 2000. PMID: 10851001 Free PMC article. - Different strategies for molecular differentiation of Mycobacterium bovis strains isolated in Sardinia, Italy.
Sechi LA, Leori G, Lollai SA, Duprè I, Molicotti P, Fadda G, Zanetti S. Sechi LA, et al. Appl Environ Microbiol. 1999 Apr;65(4):1781-5. doi: 10.1128/AEM.65.4.1781-1785.1999. Appl Environ Microbiol. 1999. PMID: 10103282 Free PMC article. - Pre-clinical development of BCG.HIVA(CAT), an antibiotic-free selection strain, for HIV-TB pediatric vaccine vectored by lysine auxotroph of BCG.
Saubi N, Mbewe-Mvula A, Gea-Mallorqui E, Rosario M, Gatell JM, Hanke T, Joseph J. Saubi N, et al. PLoS One. 2012;7(8):e42559. doi: 10.1371/journal.pone.0042559. Epub 2012 Aug 21. PLoS One. 2012. PMID: 22927933 Free PMC article.
References
- Abou-Zeid C, Smith I, Grange J, Steele J, Rook G. Subdivision of daughter strains of bacille Calmette-Guérin (BCG) according to secreted protein patterns. J Gen Microbiol. 1986;132:3047–3053. - PubMed
- Brosman S A. Bacillus Calmette-Guérin immunotherapy. Urol Clin N Am. 1992;19:557–564. - PubMed
- Calmette A, Guérin G, Nègre L, Boquet A. Prémunition des nouveaux-nés contre la tuberculose par le vaccin BCG, 1921–1926. Ann Inst Pasteur (Paris) 1926;40:89–133.
- Edwards K M, Kernolde D S. Possible hazards of routine bacillus Calmette-Guérin immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1996;15:836–838. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources