DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone - PubMed (original) (raw)
DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone
B Balasubramanian et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Despite extensive study, there is little experimental information available as to which of the deoxyribose hydrogen atoms of duplex DNA reacts most with the hydroxyl radical. To investigate this question, we prepared a set of double-stranded DNA molecules in which deuterium had been incorporated specifically at each position in the deoxyribose of one of the four nucleotides. We then measured deuterium kinetic isotope effects on the rate of cleavage of DNA by the hydroxyl radical. These experiments demonstrate that the hydroxyl radical reacts with the various hydrogen atoms of the deoxyribose in the order 5' H > 4' H > 3' H approximately 2' H approximately 1' H. This order of reactivity parallels the exposure to solvent of the deoxyribose hydrogens. Our work therefore reveals the structural basis of the reaction of the hydroxyl radical with DNA. These results also provide information on the mechanism of DNA damage caused by ionizing radiation as well as atomic-level detail for the interpretation of hydroxyl radical footprints of DNA-protein complexes and chemical probe experiments on the structure of RNA and DNA in solution.
Figures
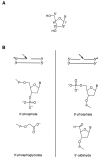
Figure 1
(A) Numbering scheme for the carbon atoms of deoxyribose. (B) Structures of the products of hydroxyl radical-mediated DNA cleavage. The asterisk indicates the position of the 32P radiolabel. (Left) Structure of the 3′ end of the DNA strand at the site of cleavage (indicated by the arrow). (Right) Structure of the 5′ end of the DNA strand at the site of cleavage (indicated by the arrow).
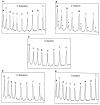
Figure 2
Comparison of hydroxyl radical cleavage patterns of control (all-protio) vs. deuterated DNA radiolabeled with 32P at the 5′ end. Broken line, control DNA. Solid line, deuterated DNA. Deuterated nucleotides are indicated by unfilled labels. The major cleavage product seen at each nucleotide is a strand terminated by a 3′-phosphate (see Fig. 1_B_); the minor product is the 3′-phosphoglycolate-terminated strand. Phosphoglycolate bands are marked in B by asterisks. (A) Control DNA vs. DNA containing 5′-dideuterated deoxycytidine. (B) Control DNA vs. DNA containing 4′-deuterated deoxythymidine. (C) Control DNA vs. DNA containing 3′-deuterated deoxyadenosine. (D) Control DNA vs. DNA containing 2′-dideuterated deoxyadenosine. (E) Control DNA vs. DNA containing 1′-deuterated deoxycytidine.
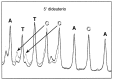
Figure 3
Comparison of hydroxyl radical cleavage patterns of control (all-protio) vs. 5′-dideuterated DNA radiolabeled with 32P at the 3′ end. Broken line, control DNA. Solid line, deuterated DNA. 5′-dideuterated deoxycytidine nucleotides are indicated by unfilled labels. The major cleavage product seen at each nucleotide is a strand terminated by a 5′-phosphate; the minor product is the 5′-aldehyde-terminated strand (see Fig. 1_B_). An aldehyde-terminated strand exhibits an unusually slow migration on the gel; previous work has established that there is a 2–3 nucleotide retardation in mobility compared with the corresponding Maxam–Gilbert product (10). Arrows show the correspondence between a 5′-phosphate-terminated product and the 5′-aldehyde-terminated product that is produced by reaction with that nucleotide.
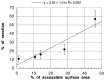
Figure 4
Solvent accessibility vs. reactivity toward the hydroxyl radical. The data in Table 2 are plotted, and the best linear fit to the data is shown. Above the graph is the equation of the best-fit line, along with the correlation coefficient.
Similar articles
- Site-specific OH attack to the sugar moiety of DNA: a comparison of experimental data and computational simulation.
Aydogan B, Marshall DT, Swarts SG, Turner JE, Boone AJ, Richards NG, Bolch WE. Aydogan B, et al. Radiat Res. 2002 Jan;157(1):38-44. doi: 10.1667/0033-7587(2002)157[0038:ssoatt]2.0.co;2. Radiat Res. 2002. PMID: 11754640 - Chemical probing of RNA with the hydroxyl radical at single-atom resolution.
Ingle S, Azad RN, Jain SS, Tullius TD. Ingle S, et al. Nucleic Acids Res. 2014 Nov 10;42(20):12758-67. doi: 10.1093/nar/gku934. Epub 2014 Oct 13. Nucleic Acids Res. 2014. PMID: 25313156 Free PMC article. - Molecular mechanisms of radiation induced DNA damage: H-abstraction and beta-cleavage.
Pardo L, Osman R, Banfelder J, Mazurek AP, Weinstein H. Pardo L, et al. Free Radic Res Commun. 1991;12-13 Pt 2:461-3. doi: 10.3109/10715769109145818. Free Radic Res Commun. 1991. PMID: 1648009 - Mechanism of radiation-induced strand break formation in DNA and polynucleotides.
Schulte-Frohlinde D. Schulte-Frohlinde D. Adv Space Res. 1986;6(11):89-96. doi: 10.1016/0273-1177(86)90281-4. Adv Space Res. 1986. PMID: 11537251 Review. - On the irrelevancy of hydroxyl radical to DNA damage from oxidative stress and implications for epigenetics.
Fleming AM, Burrows CJ. Fleming AM, et al. Chem Soc Rev. 2020 Sep 21;49(18):6524-6528. doi: 10.1039/d0cs00579g. Chem Soc Rev. 2020. PMID: 32785348 Free PMC article. Review.
Cited by
- Analysis of the Abasic Sites in Breast Cancer Patients With 5 Year Postoperative Treatment Without Recurrence in Taiwan.
Lin C, Feng CY, Bahari GP, Huang SM, Wei CH, Xu Q, Dinh DT, Chen DR, Lin PH. Lin C, et al. Cancer Control. 2024 Jan-Dec;31:10732748241300656. doi: 10.1177/10732748241300656. Cancer Control. 2024. PMID: 39520259 Free PMC article. - Effect of Dental Local Anesthetics on Reactive Oxygen Species: An In Vitro Study.
Kuroda H, Tsukimoto S, Kosai A, Komatsu N, Ouchi T, Kimura M, Sato-Boku A, Yoshida A, Yoshino F, Abe T, Shibukawa Y, Sanuki T. Kuroda H, et al. Cureus. 2024 Jun 29;16(6):e63479. doi: 10.7759/cureus.63479. eCollection 2024 Jun. Cureus. 2024. PMID: 39077267 Free PMC article. - Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination.
Boros LG, Kyriakopoulos AM, Brogna C, Piscopo M, McCullough PA, Seneff S. Boros LG, et al. Pharmacol Res Perspect. 2024 Jun;12(3):e1218. doi: 10.1002/prp2.1218. Pharmacol Res Perspect. 2024. PMID: 38867495 Free PMC article. Review. - Aging-Induced Reduction in Safflower Seed Germination via Impaired Energy Metabolism and Genetic Integrity Is Partially Restored by Sucrose and DA-6 Treatment.
Lv T, Li J, Zhou L, Zhou T, Pritchard HW, Ren C, Chen J, Yan J, Pei J. Lv T, et al. Plants (Basel). 2024 Feb 27;13(5):659. doi: 10.3390/plants13050659. Plants (Basel). 2024. PMID: 38475505 Free PMC article. - Air Pollution and Lung Cancer: Contributions of Extracellular Vesicles as Pathogenic Mechanisms and Clinical Utility.
González-Ruíz J, A Baccarelli A, Cantu-de-Leon D, Prada D. González-Ruíz J, et al. Curr Environ Health Rep. 2023 Dec;10(4):478-489. doi: 10.1007/s40572-023-00421-8. Epub 2023 Dec 6. Curr Environ Health Rep. 2023. PMID: 38052753 Free PMC article. Review.
References
- von Sonntag C. The Chemical Basis of Radiation Biology. London: Taylor & Francis; 1987.
- Tullius T D. Nature (London) 1988;332:663–664. - PubMed
- Lee S, Hahn S. Nature (London) 1995;376:609–612. - PubMed
- Price M A, Tullius T D. Methods Enzymol. 1992;212:194–219. - PubMed
- Latham J A, Cech T R. Science. 1989;245:276–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources