Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase - PubMed (original) (raw)
Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase
X Cheng et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Although phosphorylation of Thr-197 in the activation loop of the catalytic subunit of cAMP-dependent protein kinase (PKA) is an essential step for its proper biological function, the kinase responsible for this reaction in vivo has remained elusive. Using nonphosphorylated recombinant catalytic subunit as a substrate, we have shown that the phosphoinositide-dependent protein kinase, PDK1, expressed in 293 cells, phosphorylates and activates the catalytic subunit of PKA. The phosphorylation of PKA by PDK1 is rapid and is insensitive to PKI, the highly specific heat-stable protein kinase inhibitor. A mutant form of the catalytic subunit where Thr-197 was replaced with Asp was not a substrate for PDK1. In addition, phosphorylation of the catalytic subunit can be monitored immunochemically by using antibodies that recognize Thr-197 phosphorylated enzyme but not unphosphorylated enzyme or the Thr197Asp mutant. PDK1, or one of its homologs, is thus a likely candidate for the in vivo PKA kinase that phosphorylates Thr-197. This finding opens a new dimension in our thinking about this ubiquitous protein kinase and how it is regulated in the cell.
Figures
Figure 1
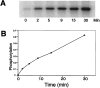
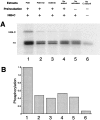
Time course of phosphorylation of the catalytic subunit of PKA by immobilized Myc-PDK1. The immunoprecipitation kinase assays were performed as described in Materials and Methods. The reactions were stopped by addition of 20 mM EDTA, and samples were analyzed by SDS/PAGE. The 32P-labeled proteins were visualized by autoradiography. Phosphorylation was quantitated by phosphoimaging. Units are arbitrary.
Figure 2
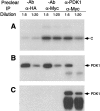
Phosphorylation of catalytic subunit of PKA by PDK1 under various conditions. The concentration of the heat stable protein kinase inhibitor, PKI, was 0.3 μM. The amount of Myc-PDK1 and Myc-PDK1-KD used was equivalent based on Western blot analysis. Positions of PDK1 and C subunit migration are indicated by arrows.
Figure 3
Myc-PDK1-mediated phosphorylation of (H89)-C is prevented by depletion of Myc-PDK1 with PDK1 antibodies. The S100 fraction of 293 cells transfected with Myc-PDK1 was diluted 5- or 20-fold and then preincubated with or without PDK1 antibodies as described in Materials and Methods. Cross-reacting proteins were removed by centrifugation after the addition of protein G beads. (A) Autoradiography showing phosphorylation of H89-C by Myc-PDK1 with or without preincubation with the PDK1 antibody. (B) Western blot showing the amount of Myc-PDK1 remaining in the cell extracts with and without preincubation with PDK1 antibody. (C) Immunoblot displaying the amount of Myc-PDK1 pulled down by pretreatment with anti PDK1.
Figure 4
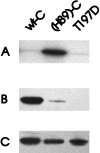
Phosphorylation of (H89)-C by PDK1 at Thr-197. (A) Phosphorylation of wild-type C, H89-C, and mutant T197D by PDK1. Phosphorylation was analyzed by SDS/PAGE followed by autoradiography. (B) Western blot analysis using antibodies specific for the phosphorylated PKC activation loop. (C) Immunoblot using anti-C subunit antibodies to show equivalent amounts of wild-type C, (H89)-C, and T197D were used in A and B.
Figure 5
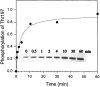
Phosphorylation of (H89)-C by PDK1 as a function of time. H89-C (0.37 μg) was incubated with total cell extracts of 293 cells expressing Myc-PDK1 (7.5 μl) in the presence of 200 μM MgATP at room temperature. The total volume of the reaction mixture was 60 μl. At different time intervals, 5 μl were withdrawn, mixed with 5 μl 2X SDS sample buffer, and heated at 100°C for 2 min to stop the reaction. The phosphorylation of Thr-197 by PDK1 was probed by Western blot by using PKC antibodies that are also specific for C subunit that is phosphorylated at Thr-197. Control experiments using nontransfected 293 cell extract showed negligible phosphorylation of (H89)-C (data not shown). Phosphorylation units are arbitrary.
Figure 6
Activation of PKA by PDK1. The S100 fractions of 293 cells (2.5 μl) independently transfected with Myc-PDK1 and Myc-PDK1-KD, as well as nontransfected 293 cells were immunoprecipitated by Myc antibodies and protein G-Sepharose as in Fig. 1. The immunoprecipitates were then mixed with histidine-tagged (H89)-C (0.125 μg) in the presence of [γ-32P]ATP. After incubation at room temperature for 30 min, the protein G beads were removed by centrifugation and H1 (1 μg) was added. The reaction mixtures were further incubated at room temperature for additional 10 min. Phosphorylated H1 was resolved by SDS/PAGE and visualized by autoradiography. Phosphorylation of H1 was quantitated by phosphoimaging. Units are arbitrary.
Figure 7
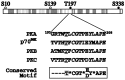
Sequence alignment of the activation loop region of PKA, PKB, PKC, and p70s6k. The specific Thr residue whose phosphorylation is important for PKA activity is underlined and identical amino acid residues are highlighted in italic. The consensus site is indicated at the bottom. The ∗ corresponds to hydrophobic residues.
Similar articles
- Phosphorylation of the catalytic subunit of protein kinase A. Autophosphorylation versus phosphorylation by phosphoinositide-dependent kinase-1.
Moore MJ, Kanter JR, Jones KC, Taylor SS. Moore MJ, et al. J Biol Chem. 2002 Dec 6;277(49):47878-84. doi: 10.1074/jbc.M204970200. Epub 2002 Oct 7. J Biol Chem. 2002. PMID: 12372837 - The activation loop of PKA catalytic isoforms is differentially phosphorylated by Pkh protein kinases in Saccharomyces cerevisiae.
Haesendonckx S, Tudisca V, Voordeckers K, Moreno S, Thevelein JM, Portela P. Haesendonckx S, et al. Biochem J. 2012 Dec 15;448(3):307-20. doi: 10.1042/BJ20121061. Biochem J. 2012. PMID: 22957732 - Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA.
Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. Biondi RM, et al. EMBO J. 2000 Mar 1;19(5):979-88. doi: 10.1093/emboj/19.5.979. EMBO J. 2000. PMID: 10698939 Free PMC article. - Physiological phosphorylation of protein kinase A at Thr-197 is by a protein kinase A kinase.
Cauthron RD, Carter KB, Liauw S, Steinberg RA. Cauthron RD, et al. Mol Cell Biol. 1998 Mar;18(3):1416-23. doi: 10.1128/MCB.18.3.1416. Mol Cell Biol. 1998. PMID: 9488457 Free PMC article. - PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle.
Dong LQ, Liu F. Dong LQ, et al. Am J Physiol Endocrinol Metab. 2005 Aug;289(2):E187-96. doi: 10.1152/ajpendo.00011.2005. Am J Physiol Endocrinol Metab. 2005. PMID: 16014356 Review.
Cited by
- Cotranslational cis-phosphorylation of the COOH-terminal tail is a key priming step in the maturation of cAMP-dependent protein kinase.
Keshwani MM, Klammt C, von Daake S, Ma Y, Kornev AP, Choe S, Insel PA, Taylor SS. Keshwani MM, et al. Proc Natl Acad Sci U S A. 2012 May 15;109(20):E1221-9. doi: 10.1073/pnas.1202741109. Epub 2012 Apr 9. Proc Natl Acad Sci U S A. 2012. PMID: 22493239 Free PMC article. - Exploring the Plasmodium falciparum cyclic-adenosine monophosphate (cAMP)-dependent protein kinase (PfPKA) as a therapeutic target.
Haste NM, Talabani H, Doo A, Merckx A, Langsley G, Taylor SS. Haste NM, et al. Microbes Infect. 2012 Aug;14(10):838-50. doi: 10.1016/j.micinf.2012.05.004. Epub 2012 May 22. Microbes Infect. 2012. PMID: 22626931 Free PMC article. Review. - Steady-state kinetic mechanism of PDK1.
Gao X, Harris TK. Gao X, et al. J Biol Chem. 2006 Aug 4;281(31):21670-21681. doi: 10.1074/jbc.M602448200. Epub 2006 May 31. J Biol Chem. 2006. PMID: 16737971 Free PMC article. - The nuts and bolts of AGC protein kinases.
Pearce LR, Komander D, Alessi DR. Pearce LR, et al. Nat Rev Mol Cell Biol. 2010 Jan;11(1):9-22. doi: 10.1038/nrm2822. Nat Rev Mol Cell Biol. 2010. PMID: 20027184 Review. - Autophosphorylation kinetics of protein kinases.
Wang ZX, Wu JW. Wang ZX, et al. Biochem J. 2002 Dec 15;368(Pt 3):947-52. doi: 10.1042/BJ20020557. Biochem J. 2002. PMID: 12190618 Free PMC article.
References
- Hunter T. Cell. 1987;50:823–829. - PubMed
- Hanks S K, Quinn A M, Hunter T. Science. 1988;241:42–52. - PubMed
- Hank S K, Hunter T. FASEB J. 1995;9:576–596. - PubMed
- Pawson T, Scott J D. Science. 1997;278:2075–2080. - PubMed
- Johnson L N, Noble M E M, Owen D J. Cell. 1996;85:149–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous