Acetylation and modulation of erythroid Krüppel-like factor (EKLF) activity by interaction with histone acetyltransferases - PubMed (original) (raw)
Acetylation and modulation of erythroid Krüppel-like factor (EKLF) activity by interaction with histone acetyltransferases
W Zhang et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Erythroid Krüppel-like factor (EKLF) is a red cell-specific transcriptional activator that is crucial for consolidating the switch to high levels of adult beta-globin expression during erythroid ontogeny. EKLF is required for integrity of the chromatin structure at the beta-like globin locus, and it interacts with a positive-acting factor in vivo. We find that EKLF is an acetylated transcription factor, and that it interacts in vivo with CBP, p300, and P/CAF. However, its interactions with these histone acetyltransferases are not equivalent, as CBP and p300, but not P/CAF, utilize EKLF as a substrate for in vitro acetylation within its trans-activation region. The functional effects of these interactions are that CBP and p300, but not P/CAF, enhance EKLF's transcriptional activation of the beta-globin promoter in erythroid cells. These results establish EKLF as a tissue-specific transcription factor that undergoes post-translational acetylation and suggest a mechanism by which EKLF is able to alter chromatin structure and induce beta-globin expression within the beta-like globin cluster.
Figures
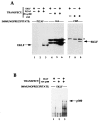
Figure 1
Tests of EKLF interaction with CBP, p300, and P/CAF in vivo. Combinations of EKLF and HAT coactivator expression plasmids (10 μg each) as indicated were transfected into COS7 cells and whole cell extracts were subjected to immunoprecipitation with anti-CBP, anti-HA, anti-P/CAF, or anti-EKLF antibodies. (A) Immunoprecipitated complexes were resolved, blotted, and probed with anti-EKLF monoclonal antibody 4B9. Location of co-electrophoresed EKLF in each gel is shown. Asterisks indicate nonspecific (Ig heavy chain) signals from the immunoprecipitating antibodies. (B) Immunoprecipitated complexes were resolved, blotted, and probed with anti-HA monoclonal antibody for detection of p300. Location of co-electrophoresed HA-p300 is shown.
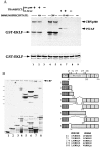
Figure 2
Tests of EKLF acetylation by CBP, p300, and P/CAF in vitro. (A) COS7 cells were transfected and extracts were immunoprecipitated with the indicated antibodies. IP-HAT assays utilized 5 μg of GST-EKLF(76–376). Samples were electrophoresed and the dried gel was exposed in autoradiography (Upper). GST-EKLF(76–376) alone was tested for autoacetylation in lane 9. Locations of p300/CBP, P/CAF, and GST-EKLF are indicated. Protein samples were also stained to show that equivalent amounts were used (Lower). (B) Endogenous CBP from COS7 cells was immunoprecipitated, and IP-HAT assays were performed with 5 μg of various GST-EKLF fusion proteins as diagrammed on the Right, which also shows the locations and sequences of lysines conserved between mouse and human EKLF [amino acid residues 47, 74, 177, 279, 288; mouse numbering is based on initiator methionine being residue 19 (9)]. Proteins were resolved and subjected to autoradiography (Upper Left) or stained for protein (Lower Left). Asterisks show the location of nondegraded GST-EKLF fusion proteins. Molecular mass markers (on the left) are 70, 55, 33, 25, and 15 kDa (top to bottom).
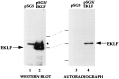
Figure 3
Status of EKLF acetylation in vivo. COS7 cells transfected with 10 μg of pSG5 or pSG5/EKLF as indicated were labeled with sodium [3H]acetate in the presence of TSA and extracts were immunoprecipitated with anti-EKLF monoclonal antibody 6B3. Immunoprecipitated samples were resolved on SDS/PAGE and blotted and probed with anti-EKLF (Left) or processed and exposed in autoradiography (Right). Asterisk indicates a nonspecific signal from the immunoprecipitating antibody. Locations of molecular mass markers (70, 50, and 33 kDa, top to bottom) and EKLF are shown.
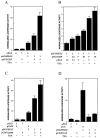
Figure 4
Analyses of EKLF transactivation of the β-globin promoter. K562 erythroleukemic cells were transfected with 1 μg of HS2/β/luc reporter and the indicated amounts (μg) of expression or vector plasmids, and extracts were processed for luciferase activity. TSA was added where indicated to a final concentration of 2 μM at the time of transfection. Multiple experiments were averaged after normalization of luciferase activity to cotransfected growth hormone levels. The effects of CBP (A), p300 (C), and P/CAF (D) are shown along with a dose–response test of CBP (B).
Similar articles
- Site-specific acetylation by p300 or CREB binding protein regulates erythroid Krüppel-like factor transcriptional activity via its interaction with the SWI-SNF complex.
Zhang W, Kadam S, Emerson BM, Bieker JJ. Zhang W, et al. Mol Cell Biol. 2001 Apr;21(7):2413-22. doi: 10.1128/MCB.21.7.2413-2422.2001. Mol Cell Biol. 2001. PMID: 11259590 Free PMC article. - Stimulation of CREB binding protein nucleosomal histone acetyltransferase activity by a class of transcriptional activators.
Chen CJ, Deng Z, Kim AY, Blobel GA, Lieberman PM. Chen CJ, et al. Mol Cell Biol. 2001 Jan;21(2):476-87. doi: 10.1128/MCB.21.2.476-487.2001. Mol Cell Biol. 2001. PMID: 11134336 Free PMC article. - Transcriptional factors for specific globin genes.
Bieker JJ, Ouyang L, Chen X. Bieker JJ, et al. Ann N Y Acad Sci. 1998 Jun 30;850:64-9. doi: 10.1111/j.1749-6632.1998.tb10463.x. Ann N Y Acad Sci. 1998. PMID: 9668528 Review. - ChIPs of the beta-globin locus: unraveling gene regulation within an active domain.
Bulger M, Sawado T, Schübeler D, Groudine M. Bulger M, et al. Curr Opin Genet Dev. 2002 Apr;12(2):170-7. doi: 10.1016/s0959-437x(02)00283-6. Curr Opin Genet Dev. 2002. PMID: 11893490 Review.
Cited by
- KLF6 Acetylation Promotes Sublytic C5b-9-Induced Production of MCP-1 and RANTES in Experimental Mesangial Proliferative Glomerulonephritis.
Yu T, Gong Y, Liu Y, Xia L, Zhao C, Liu L, Xie M, Wu Z, Zhao D, Qiu W, Wang Y, Zhang J, Ji M. Yu T, et al. Int J Biol Sci. 2020 Jun 20;16(13):2340-2356. doi: 10.7150/ijbs.46573. eCollection 2020. Int J Biol Sci. 2020. PMID: 32760202 Free PMC article. - Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway.
Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ, Ratan RR. Ryu H, et al. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4281-6. doi: 10.1073/pnas.0737363100. Epub 2003 Mar 14. Proc Natl Acad Sci U S A. 2003. PMID: 12640146 Free PMC article. - A tale of three fingers: the family of mammalian Sp/XKLF transcription factors.
Philipsen S, Suske G. Philipsen S, et al. Nucleic Acids Res. 1999 Aug 1;27(15):2991-3000. doi: 10.1093/nar/27.15.2991. Nucleic Acids Res. 1999. PMID: 10454592 Free PMC article. Review. - Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia.
Lavau C, Du C, Thirman M, Zeleznik-Le N. Lavau C, et al. EMBO J. 2000 Sep 1;19(17):4655-64. doi: 10.1093/emboj/19.17.4655. EMBO J. 2000. PMID: 10970858 Free PMC article. - An embryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer.
Tanabe O, Katsuoka F, Campbell AD, Song W, Yamamoto M, Tanimoto K, Engel JD. Tanabe O, et al. EMBO J. 2002 Jul 1;21(13):3434-42. doi: 10.1093/emboj/cdf340. EMBO J. 2002. PMID: 12093744 Free PMC article.
References
- Townes T M, Behringer R R. Trends Genet. 1990;6:219–223. - PubMed
- Felsenfeld G. Nature (London) 1992;355:219–224. - PubMed
- Engel J D. Trends Genet. 1993;9:304–309. - PubMed
- Orkin S H. Eur J Biochem. 1995;231:271–281. - PubMed
- Martin D I K, Fiering S, Groudine M. Curr Opin Genet Dev. 1996;6:488–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous