cyclops encodes a nodal-related factor involved in midline signaling - PubMed (original) (raw)
cyclops encodes a nodal-related factor involved in midline signaling
M R Rebagliati et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Ventral structures in the central nervous system are patterned by signals emanating from the underlying mesoderm as well as originating within the neuroectoderm. Mutations in the zebrafish, Danio rerio, are proving instrumental in dissecting these midline signals. The cyclops mutation leads to a loss of medial floor plate and to severe deficits in ventral forebrain development, leading to cyclopia. Here, we report that the cyclops locus encodes the nodal-related protein Ndr2, a member of the transforming growth factor type beta superfamily of factors. The evidence includes identification of a missense mutation in the initiation codon and rescue of the cyclops phenotype by expression of ndr2 RNA, here renamed "cyclops." Thus, in interaction with other molecules implicated in these processes such as sonic hedgehog and one-eyed-pinhead, cyclops is required for ventral midline patterning of the embryonic central nervous system.
Figures
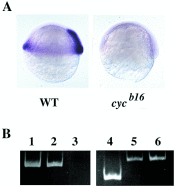
Figure 1
(A) ndr2 RNA is present in wild-type (WT) but not homozygous _cyc_b16 embryos, as seen by in situ hybridization at the shield stage (28). (B) ndr2 is deleted in _cyc_b16 DNA, visualized by PCR. Lanes: 1, ndr2 primer pair 1 + ndr2 plasmid; 2, ndr2 primer pair 1 + wild-type genomic DNA; 3, ndr2 primer pair 1 + _cyc_b16 homozygous genomic DNA; 4, ndr2 primer pair 2 + ndr2 plasmid; 5, ndr1 primers + ndr2 primer pair 2 + _cyc_b16 genomic DNA; 6, ndr1 primers + ndr1 plasmid.
Figure 2
(A) ndr2 is linked to _cyc_tf219, as shown by segregation of an Aci I restriction polymorphism in ndr2 sequences (16) amplified from genomic DNAs of a mapping cross. Lanes: 1, heterozygous _cyc_tf219 G0 fish; 2, homozygous wild-type G0 fish (WIK strain); 3, F2 mutant sibs (homozygous _cyc_tf219); 4, F2 phenotypically wild-type (heterozygous and homozygous wild-type) sibs. Arrow denotes fragment generated by Aci I site present in the WIK but not the _cyc_tf219 strain; this restriction fragment length polymorphism segregates with the cyc mutation, demonstrating linkage. (B) The first methionine of the ndr2 ORF is changed to isoleucine in _cyc_tf219; the M→I mutation was verified in two independent amplification products. In the schematic drawing, shading denotes region of mature Ndr2 polypeptide (382–501). (C) Translation of ndr2 sequence showing the first 61 aa of the ORF (upper case). Asterisk denotes the first in-frame stop codon upstream of the initiating methionine. (D) Hydrophobicity plot of the N-terminal region of Ndr2, generated with the
pepplot
program of the GCG package; green and red denote hydrophobic and hydrophilic regions, respectively.
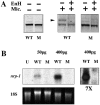
Figure 3
(A) The Ndr2 protein encoded by the _cyc_tf219 allele lacks a functional signal sequence, as judged by the failure to be glycosylated. Proteins from coupled transcription–translation reactions with expression plasmids that initiate at the wild-type met (WT) or the second met at position 38 (corresponding to _cyc_tf219, denoted M) were analyzed under denaturing and reducing conditions. Aliquots were treated with or without dog pancreatic microsomes (Mic.) and±endoglycosidase H. The arrow indicates the glycosylated form of the polypeptide. (B) Northern blot analysis of the in vivo activity of wild-type (WT) and mutant (M) RNA in the Xenopus animal cap assay, using nrp-1 as a probe for neural induction (28). U, uninjected; pg, amount of RNA injected per embryo; 18S rRNA was used as a loading control; 7×, sevenfold longer exposure of the two lanes at the right to illustrate the inactivity of the mutant RNA.
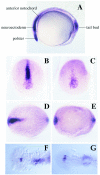
Figure 4
The expression pattern of cyc at the bud stage is modified in _cyc_tf219 mutant embryos. (A) The wild-type pattern (28). Cyc RNA is seen in the tailbud, and the anterior notochord and prechordal plate including the polster (hatching gland precursor). (B, C, and F) Wild-type sibs. (D, E, and G) _cyc_tf219 mutant embryos. Anterior views (B and D) and dorsal views (C_–_G) are shown. At the tailbud stage, expression in tailbud and polster is essentially unaffected in _cyc_tf219, but expression in the prechordal plate, ventral neuroectoderm, and anterior notochord is largely lost. At the 20-somite stage, asymmetry of staining in the lateral plate and diencephalon is lost in mutant embryos.
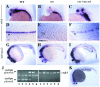
Figure 5
Rescue of the _cyc_b16 phenotype by injection of a cyc expression construct. Wild-type (A, D, and G), cyc (B, E, and H), and cyc rescued by injection of 0.5 pg DNA per embryo (C, F, I, and K) are shown, as hybridized with twhh (A_–_F and K) or shh and pax2 (G_–_I). (J) After staining, the embryos were genotyped by PCR with primers that span an intron in the cyc gene; injected embryos also showed amplification product from the intronless expression plasmid. Aliquots of the same DNA samples also were amplified with primers for the unlinked ndr1 gene as a positive control. Lanes: 1, amplification of DNA from embryo in C; 2, F; 3, I; 4, injected wild type; 5, uninjected wild type; 6, uninjected cyc.
Similar articles
- Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry.
Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB. Rebagliati MR, et al. Dev Biol. 1998 Jul 15;199(2):261-72. doi: 10.1006/dbio.1998.8935. Dev Biol. 1998. PMID: 9698446 - Analysis of novel domain-specific mutations in the zebrafish ndr2/cyclops gene generated using CRISPR-Cas9 RNPs.
Turner AN, Andersen RS, Bookout IE, Brashear LN, Davis JC, Gahan DM, Davis JC, Gotham JP, Hijaz BA, Kaushik AS, Mcgill JB, Miller VL, Moseley ZP, Nowell CL, Patel RK, Rodgers MC, Patel RK, Shihab YA, Walker AP, Glover SR, Foster SD, Challa AK. Turner AN, et al. J Genet. 2018 Dec;97(5):1315-1325. J Genet. 2018. PMID: 30555080 - A temperature-sensitive mutation in the nodal-related gene cyclops reveals that the floor plate is induced during gastrulation in zebrafish.
Tian J, Yam C, Balasundaram G, Wang H, Gore A, Sampath K. Tian J, et al. Development. 2003 Jul;130(14):3331-42. doi: 10.1242/dev.00544. Development. 2003. PMID: 12783802 - [Axis formation in zebrafish].
Hibi M, Shimizu T, Hashimoto H, Ryu SL, Yamanaka Y, Hirano T. Hibi M, et al. Tanpakushitsu Kakusan Koso. 2000 Dec;45(17 Suppl):2720-31. Tanpakushitsu Kakusan Koso. 2000. PMID: 11187772 Review. Japanese. No abstract available. - Zebrafish hearts and minds: nodal signaling in cardiac and neural left-right asymmetry.
Long S, Ahmad N, Rebagliati M. Long S, et al. Cold Spring Harb Symp Quant Biol. 2002;67:27-36. doi: 10.1101/sqb.2002.67.27. Cold Spring Harb Symp Quant Biol. 2002. PMID: 12858520 Review. No abstract available.
Cited by
- Understanding laterality disorders and the left-right organizer: Insights from zebrafish.
Forrest K, Barricella AC, Pohar SA, Hinman AM, Amack JD. Forrest K, et al. Front Cell Dev Biol. 2022 Dec 23;10:1035513. doi: 10.3389/fcell.2022.1035513. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36619867 Free PMC article. Review. - Light-activated Frizzled7 reveals a permissive role of non-canonical wnt signaling in mesendoderm cell migration.
Čapek D, Smutny M, Tichy AM, Morri M, Janovjak H, Heisenberg CP. Čapek D, et al. Elife. 2019 Jan 16;8:e42093. doi: 10.7554/eLife.42093. Elife. 2019. PMID: 30648973 Free PMC article. - Shp2 knockdown and Noonan/LEOPARD mutant Shp2-induced gastrulation defects.
Jopling C, van Geemen D, den Hertog J. Jopling C, et al. PLoS Genet. 2007 Dec;3(12):e225. doi: 10.1371/journal.pgen.0030225. PLoS Genet. 2007. PMID: 18159945 Free PMC article. - Zebrafish and medaka: model organisms for a comparative developmental approach of brain asymmetry.
Signore IA, Guerrero N, Loosli F, Colombo A, Villalón A, Wittbrodt J, Concha ML. Signore IA, et al. Philos Trans R Soc Lond B Biol Sci. 2009 Apr 12;364(1519):991-1003. doi: 10.1098/rstb.2008.0260. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19064351 Free PMC article. - Temperature Sensitivity of Neural Tube Defects in Zoep Mutants.
Ma P, Swartz MR, Kindt LM, Kangas AM, Liang JO. Ma P, et al. Zebrafish. 2015 Dec;12(6):448-56. doi: 10.1089/zeb.2015.1113. Epub 2015 Sep 14. Zebrafish. 2015. PMID: 26366681 Free PMC article.
References
- Sasai Y, De Robertis E M. Dev Biol. 1997;182:5–20. - PubMed
- Hemmati-Brivanlou A, Melton D. Annu Rev Neurosci. 1997;20:43–60. - PubMed
- Placzek M, Furley A. Curr Biol. 1996;6:526–529. - PubMed
- Placzek M, Tessier-Lavigne M, Yamada T, Jessell T, Dodd J. Science. 1990;250:985–988. - PubMed
- Clarke J D, Holder N, Soffe S R, Storm-Mathisen J. Development. 1991;112:499–516. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases