A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice - PubMed (original) (raw)
A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice
C Ma et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Cyclin D1-deficient mice have small eyes with thin retinas. We observed that there was a lower level of retinal cell proliferation and a unique pattern of photoreceptor cell death. Death was first observed in scattered clusters of cells in the retina. It then appeared to spread from these few cells to nearby photoreceptors, eventually producing extensive holes in the photoreceptor layer. These holes appeared to be filled with interneurons from the inner nuclear layer. The death mainly occurred during the second to fourth postnatal weeks. Other models of photoreceptor degeneration in rodents differ in that they occur more uniformly across the retina, with death proceeding over a longer period of time until all, or nearly all, of the photoreceptors degenerate. We also tested whether expression of a bcl-2 transgene could prevent the death and found that it could not.
Figures
Figure 1
Reduction in mitosis and total cell number in cyclin D1 mutant retinas. (A) Neonatal (P0) retinas were labeled for 12 hr by [3H]thymidine, and the percentages of 3H-labeled retinal cells were scored. (B) The total number of retinal cells in mice of the indicated ages. Error bars show SD. The numbers in parentheses indicate the number of animals examined.
Figure 2
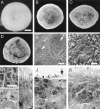
Cell death in the cyclin D1 mutant retinas. (A_–_D) Dark field images of intact retinas. (A) Wild-type, P10. (B) _Cyclin D1_−/−, P10. (C) _Cyclin D1_−/−, P21. (C) _Cyclin D1_−/−, P180. (E and F) Scanning electron microscopy images of the outer segment surface of a P21 _cyclin D1_−/− retina. (F) High magnification view of the area indicated by the arrowhead in E. Small arrow in E points to a small cluster of cells that protrude into the outer segment layer. Arrowhead in E points to a large cluster of cells that appear to be in an expanding hole. Large arrow in E points to a large hole. (G_–_K) TEM images of retinal sections. (G_–_I) P21 wild-type retina. (G) Nuclei in the ONL. (H) Nuclei in the INL. (I) Inner and outer segments of photoreceptors. (J and K), P21 _cyclin D1_−/− retina. (J) A hole (indicated by the large arrow) and the boundary of the hole (indicated by the dashed line). Note the presence of cells with INL cell morphology (small arrow) in the hole at the ONL level and the photoreceptor cells without inner or outer segments (arrowhead) at the boundary of the hole. (K) An area that is not a hole. Note the presence of many pale nuclei in the ONL (arrow) and reduction in the length of the inner and outer segments of the photoreceptors. OPL, outer plexiform layer. OS, outer segment. IS, inner segment. (Scale bar in A is 500 μm; A_–_D are to the same scale. Scale bar in E is 20 μm. Scale bar in F is 5 μm. Scale bar in G is 5 μm; and G_–_K are to the same scale.)
Figure 3
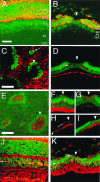
Immunohistochemical characterization of holes in the cyclin D1 mutant retinas. (A and J) Sections of P21 wild-type retinas. (B, D, F_–_I, and K) Sections of P21 _cyclin D1_−/− retinas. (C and E) Whole mount of P21 _cyclin D1_−/− retinas. Red, signals of antibody staining; green, nuclei. Arrowheads point to examples of holes. (A and B) Anti-recoverin; note the absence of recoverin-positive cells in the hole B. (C) Anti-rhodopsin, showing the outer segment surface of a mutant retina; note the absence of rhodopsin inside the holes. The arrow points to a small cell cluster that protrudes into the outer segment layer. (D) Anti-neurofilament; note the absence of neurofilament-positive cells in the hole. The arrow points to the neurofilament-positive horizontal cells outside the hole. (E_–_I) VC1.1 antibody; note the high density of VC1.1-positive cells inside the holes and reduction of VC1.1-positive cells adjacent to the holes. (E) A whole mount retina stained with VC1.1 antibody. (F_–_I) VC1.1 antibody staining of sections. (F) A hole at early stage. (G) A hole at late stage. (H) VC1.1 antibody staining of a hole at low magnification. Arrow in H points to the VC1.1-positive cells at a distance to the hole. Bracket in H indicates the area that contains fewer VC1.1-positive cells. (I) Merged image of the VC1.1 antibody staining in H and the nuclear stain. (J and K), anti-CRALBP; note the location of the CRALBP-positive Müller glial cells in the middle of the INL in the wild-type (arrow in J) and the abnormal location of the Müller glial cells in the ONL in the _cyclin D1_−/− mutant (arrows in K). OPL, outer plexiform layer. IPL, inner plexiform layer. GCL, ganglion cell layer. (Scale bar in A is 50 μm; A, B, J, and K are to the same scale. Scale bar in C is 50 μm; C, D, F, and G are to the same scale. Scale bar in E is 25 μm; E, H, and I are to the same scale.)
Figure 4
Apoptosis in the _cyclin D1_−/− mutant retinas. In all panels, the photoreceptor side of the whole mount retina is shown (vitreous side facing down). (A_–_C) Acridine orange staining. (A) _Cyclin D1_−/−, P6. Arrow points to a cluster of acridine orange-positive cells. (B) _Cyclin D1_−/−, P10; note the rings of acridine orange-positive cells and some scattered acridine orange-positive cells. (C) Wild-type. Asterisks indicate the optic nerve head. (D_–_F) TUNEL assays of P21 _cyclin D1_−/− mutant retinas. (D) Dark field image of the retina. (E) TUNEL assay viewed under UV illumination; note the presence of TUNEL-positive cells at the edge of the holes. One of the holes is indicated by the arrow and is shown at a higher magnification in F. (G and H) Negative control for TUNEL assay (without adding terminal transferase in the assay). (G) Dark field image. (H) UV illumination. (I) P10 _cyclin D1_−/−; opsin/bcl-2. Note the presence of holes. Black speckles in retinas were cells or pigment granules from the pigmented epithelium that adhered to the retinas. (Scale bar in A is 100 μm. Scale bar in B is 250 μm; B, C, D, E, G, and H are to the same scale. Scale bar in F is 50 μm. Scale bar in I is 500 μm.)
References
- Dowling J E. The Retina–An Approachable Part of the Brain. Cambridge, MA: Harvard Univ. Press; 1987.
- Young R. J Comp Neurol. 1984;229:362–373. - PubMed
- Voyvodic J T, Burne J F, Raff M C. Eur J Neurosci. 1995;7:2469–2478. - PubMed
- Humphries M H, Rancourt D, Farrar G J, Kenna P, Hazel M, Bush R A, Sieving P A, Sheils D M, McNally N, Creighton P, et al. Nat Genet. 1997;15:216–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY 6891/EY/NEI NIH HHS/United States
- EY 10992/EY/NEI NIH HHS/United States
- EY08064/EY/NEI NIH HHS/United States
- R01 EY008064/EY/NEI NIH HHS/United States
- R01 EY006891/EY/NEI NIH HHS/United States
- F32 EY006891/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials