Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure - PubMed (original) (raw)
Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure
J W Adams et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Receptor-mediated Gq signaling promotes hypertrophic growth of cultured neonatal rat cardiac myocytes and is postulated to transduce in vivo cardiac pressure overload hypertrophy. Although initially compensatory, hypertrophy can proceed by unknown mechanisms to cardiac failure. We used adenoviral infection and transgenic overexpression of the alpha subunit of Gq to autonomously activate Gq signaling in cardiomyocytes. In cultured cardiac myocytes, overexpression of wild-type Galphaq resulted in hypertrophic growth. Strikingly, expression of a constitutively activated mutant of Galphaq, which further increased Gq signaling, produced initial hypertrophy, which rapidly progressed to apoptotic cardiomyocyte death. This paradigm was recapitulated during pregnancy in Galphaq overexpressing mice and in transgenic mice expressing high levels of wild-type Galphaq. The consequence of cardiomyocyte apoptosis was a transition from compensated hypertrophy to a rapidly progressive and lethal cardiomyopathy. Progression from hypertrophy to apoptosis in vitro and in vivo was coincident with activation of p38 and Jun kinases. These data suggest a mechanism in which moderate levels of Gq signaling stimulate cardiac hypertrophy whereas high level Gq activation results in cardiomyocyte apoptosis. The identification of a single biochemical stimulus regulating cardiomyocyte growth and death suggests a plausible mechanism for the progression of compensated hypertrophy to decompensated heart failure.
Figures
Figure 1
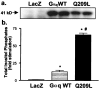
Adenovirus-mediated Gαq expression and signaling in cultured cardiac myocytes. (a) Expression of Gαq in neonatal rat cardiac myocytes (representative Western blot of three separate experiments). (b) Overexpression of Gαq stimulates phosphoinositide hydrolysis. P < 0.01 (∗) compared with LacZ; P < 0.001 (#) compared with Gαq WT (n = 9).
Figure 2
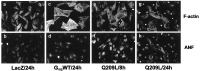
Stimulation of myocyte hypertrophy and death by Gαq overexpression. Myocytes infected with indicated adenovirus constructs were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin or polyclonal ANF antiserum. Myocyte hypertrophy is evidenced by increased ANF expression, increased sarcomeric structure, and increased cell size in Gαq-expressing cells at 8 hr. At 24 hr, Q209L expressing cells show shrinkage and death.
Figure 3
Constitutive activation of Gαq signaling causes apoptosis in myocytes. (a) In situ DNA end labeling (ISEL) of myocytes infected with indicated adenovirus constructs. ISEL positive myocytes have reddish brown nuclei. (b) Quantitative analysis of ISEL studies (n = 200 cells per experimental group from three separate experiments). P < 0.001 (∗) compared with LacZ; P < 0.001 (#) compared with Gαq WT. (c) DNA was isolated from myocytes 48 hr after infection with the indicated adenovirus constructs. DNA fragmentation was detected by ethidium bromide fluorescence.
Figure 4
Characteristics of peripartum cardiomyopathy in Gαq-overexpressing mice. (a) Survival curve relating mortality to number of pregnancies. (Inset) Chronology of heart failure development relative to parturition defined as within 7 days of expected delivery (antepartum), within 7 days after delivery (early postpartum), or 7–14 days after delivery (delayed post). (b) Gross morphology (4×) of peripartum nontransgenic (Left) and Gαq-overexpressing (Right) mouse hearts showing cardiomyopathic dilatation of cardiac chambers (LV, left ventricle; RV, right ventricle) with mural thrombi in atria (arrows). (c) Trichrome stain (400×) of hearts depicted in b shows interstitial fibrosis and myocyte replacement (blue-stained cells), without inflammation in transgenic heart (Right).
Figure 5
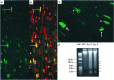
DNA strand breaks in cardiac myocyte nuclei of Gαq peripartal hearts. (a) Low power (200×) and (c) high power (1,000×) images showing nuclear DNA labeling visualized by fluorescein fluorescence. (b) a counterstained with propidium iodide (red). Apoptotic nuclei appear green or yellow (arrows), occur only in cardiac myocytes, and demonstrate varying degrees of condensed nuclear chromatin (arrows in c). Normal myocyte nuclei are red with a diffuse chromatin staining pattern. (d) DNA laddering of Gαq cardiac DNA. MW, DNA size markers.
Figure 6
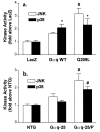
JNK and p38 MAP kinase activities in Gαq-overexpressing cardiomyocytes (a) and transgenic mice (b). There is coactivation of both kinases in apoptotic Q209L expressing cardiomyocytes and peripartum (Gαq-25/P) cardiomyopathic mice. P < 0.05 (∗) compared with control (LacZ infected) myocytes or control nontransgenic (NTG) mice, and P < 0.01 (#) compared with control (LacZ, NTG) or Gαq WT (Gαq WT, Gαq-25) groups (n = 6–9 determinations).
Similar articles
- Mitochondrial reprogramming induced by CaMKIIδ mediates hypertrophy decompensation.
Westenbrink BD, Ling H, Divakaruni AS, Gray CB, Zambon AC, Dalton ND, Peterson KL, Gu Y, Matkovich SJ, Murphy AN, Miyamoto S, Dorn GW 2nd, Heller Brown J. Westenbrink BD, et al. Circ Res. 2015 Feb 27;116(5):e28-39. doi: 10.1161/CIRCRESAHA.116.304682. Epub 2015 Jan 20. Circ Res. 2015. PMID: 25605649 Free PMC article. - Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure.
De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW 2nd, Kitsis RN, Molkentin JD. De Windt LJ, et al. Circ Res. 2000 Feb 18;86(3):255-63. doi: 10.1161/01.res.86.3.255. Circ Res. 2000. PMID: 10679475 - ROCK1 plays an essential role in the transition from cardiac hypertrophy to failure in mice.
Shi J, Zhang YW, Yang Y, Zhang L, Wei L. Shi J, et al. J Mol Cell Cardiol. 2010 Nov;49(5):819-28. doi: 10.1016/j.yjmcc.2010.08.008. Epub 2010 Aug 13. J Mol Cell Cardiol. 2010. PMID: 20709073 Free PMC article. - Gq signaling in cardiac adaptation and maladaptation.
Dorn GW 2nd, Brown JH. Dorn GW 2nd, et al. Trends Cardiovasc Med. 1999 Jan-Feb;9(1-2):26-34. doi: 10.1016/s1050-1738(99)00004-3. Trends Cardiovasc Med. 1999. PMID: 10189964 Review. - G-proteins in growth and apoptosis: lessons from the heart.
Adams JW, Brown JH. Adams JW, et al. Oncogene. 2001 Mar 26;20(13):1626-34. doi: 10.1038/sj.onc.1204275. Oncogene. 2001. PMID: 11313910 Review.
Cited by
- IGF-2R-Gαq signaling and cardiac hypertrophy in the low-birth-weight lamb.
Wang KC, Tosh DN, Zhang S, McMillen IC, Duffield JA, Brooks DA, Morrison JL. Wang KC, et al. Am J Physiol Regul Integr Comp Physiol. 2015 Apr 1;308(7):R627-35. doi: 10.1152/ajpregu.00346.2014. Epub 2015 Jan 28. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25632020 Free PMC article. - Neurohormonal activation in heart failure with reduced ejection fraction.
Hartupee J, Mann DL. Hartupee J, et al. Nat Rev Cardiol. 2017 Jan;14(1):30-38. doi: 10.1038/nrcardio.2016.163. Epub 2016 Oct 6. Nat Rev Cardiol. 2017. PMID: 27708278 Free PMC article. Review. - Cardiac hypertrophy and heart failure development through Gq and CaM kinase II signaling.
Mishra S, Ling H, Grimm M, Zhang T, Bers DM, Brown JH. Mishra S, et al. J Cardiovasc Pharmacol. 2010 Dec;56(6):598-603. doi: 10.1097/FJC.0b013e3181e1d263. J Cardiovasc Pharmacol. 2010. PMID: 20531218 Free PMC article. Review. - G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes.
Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams JW, Brown JH, Olefsky JM. Imamura T, et al. Mol Cell Biol. 1999 Oct;19(10):6765-74. doi: 10.1128/MCB.19.10.6765. Mol Cell Biol. 1999. PMID: 10490615 Free PMC article. - The human heart: a self-renewing organ.
Kajstura J, Hosoda T, Bearzi C, Rota M, Maestroni S, Urbanek K, Leri A, Anversa P. Kajstura J, et al. Clin Transl Sci. 2008 May;1(1):80-6. doi: 10.1111/j.1752-8062.2008.00030.x. Clin Transl Sci. 2008. PMID: 20443822 Free PMC article.
References
- Simon M I, Strathmann M P, Gautam N. Science. 1991;252:802–808. - PubMed
- Post G R, Brown J H. FASEB J. 1996;10:741–749. - PubMed
- Sadoshima J-I, Izumo S. Circ Res. 1993;73:413–423. - PubMed
- Shubeita H E, McDonough P M, Harris A N, Knowlton K U, Glembotski C C, Brown J H, Chien K R. J Biol Chem. 1990;265:20555–20562. - PubMed
- Knowlton K U, Michel M C, Itani M, Shubeita H E, Ishihara K, Brown J H, Chien K R. J Biol Chem. 1993;268:15374–15380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL52318/HL/NHLBI NIH HHS/United States
- P50 HL052318/HL/NHLBI NIH HHS/United States
- R01 HL058010/HL/NHLBI NIH HHS/United States
- P01 HL046345/HL/NHLBI NIH HHS/United States
- HL49267/HL/NHLBI NIH HHS/United States
- R37 HL028143/HL/NHLBI NIH HHS/United States
- HL58010/HL/NHLBI NIH HHS/United States
- R01 HL028143/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous