A novel function of the DNA repair gene rhp6 in mating-type silencing by chromatin remodeling in fission yeast - PubMed (original) (raw)
A novel function of the DNA repair gene rhp6 in mating-type silencing by chromatin remodeling in fission yeast
J Singh et al. Mol Cell Biol. 1998 Sep.
Abstract
Recent studies have indicated that the DNA replication machinery is coupled to silencing of mating-type loci in the budding yeast Saccharomyces cerevisiae, and a similar silencing mechanism may operate in the distantly related yeast Schizosaccharomyces pombe. Regarding gene regulation, an important function of DNA replication may be in coupling of faithful chromatin assembly to reestablishment of the parental states of gene expression in daughter cells. We have been interested in isolating mutants that are defective in this hypothesized coupling. An S. pombe mutant fortuitously isolated from a screen for temperature-sensitive growth and silencing phenotype exhibited a novel defect in silencing that was dependent on the switching competence of the mating-type loci, a property that differentiates this mutant from other silencing mutants of S. pombe as well as of S. cerevisiae. This unique mutant phenotype defined a locus which we named sng1 (for silencing not governed). Chromatin analysis revealed a switching-dependent unfolding of the donor loci mat2P and mat3M in the sng1(-) mutant, as indicated by increased accessibility to the in vivo-expressed Escherichia coli dam methylase. Unexpectedly, cloning and sequencing identified the gene as the previously isolated DNA repair gene rhp6. RAD6, an rhp6 homolog in S. cerevisiae, is required for postreplication DNA repair and ubiquitination of histones H2A and H2B. This study implicates the Rad6/rhp6 protein in gene regulation and, more importantly, suggests that a transient window of opportunity exists to ensure the remodeling of chromatin structure during chromosome replication and recombination. We propose that the effects of the sng1(-)/rhp6(-) mutation on silencing are indirect consequences of changes in chromatin structure.
Figures
FIG. 1
Cassette organization of the mating-type loci mat1, mat2P, and mat3M in S. pombe. All three cassettes contain the homology regions defined by H2 (135 bp) and H1 (59 bp), which are located at the two flanks of the Plus-specific (jagged line) and Minus-specific (straight line) regions. H3, the third region of homology, is present only at mat2 and mat3 loci. The arrows indicate the directional switching of Plus to Minus and Minus to Plus by transfer of information from mat2 and mat3 to mat1 by gene conversion-mediated transposition. The site of the in vivo DSB at the H1/allele junction in mat1 is indicated. The gene products of swi6, rik1, and clr1-4 are shown to be involved in silencing of the mat2 and mat3 loci. The locations of the four _cis_-acting silencer elements (I to IV) flanking the silent cassette mat2P are also indicated (14). The locations of oligonucleotide (oligo) 1, used for primer extension to detect the Pc and Mi transcripts from the mat1 locus (mat1Pc mRNA and mat1Mi RNA) for the experiment in Fig. 4, and those used to detect the RT-PCR products for mat2Pc (oligonucleotides 2Pc and Pc) and mat3Mi (oligonucleotides 3M and Mi) mRNAs for the experiment in Fig. 3 are indicated. The central 4.3-kb segment of the K region and some flanking regions which show strong homology to the centromeric sequences of cen2 and cen3 (26) are also indicated.
FIG. 2
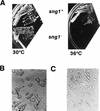
Different phenotypes of the _sng1_− mutant. (A) Temperature sensitivity. Strains used were wild-type strain SP837 (h90 leu1-32 ura4D18 ade6-M216) and mutant strain SPJ107 (h90 leu1-32 ura4D18 ade6-M216 sng1−). Temperatures at which strains were grown are indicated. h90 denotes homothallic strains that undergo sng1-1 efficient mating-type switching. (B) Haploid meiosis. The _sng1_− mutant cells exhibiting haploid meiosis are indicated by arrows. Wild-type cells produce asci containing four mature spores in zygotic diploid cells which result from switching and mating of cells of opposite mating type. In contrast, the _sng1_− mutant cells produce aberrant spores in a haploid cell.
FIG. 3
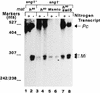
RT-PCR analysis of transcripts encoded by the donor loci. (A) mat2Pc; (B) mat3Mi; (C) polα. RNA was isolated from cells grown at the semipermissive temperature of 30°C in N+ (+; lanes 1, 3, 5, and 7) or N− (−; lanes 2, 4, 6, and 8) medium, a regimen that induces mat transcripts (31). RNA samples (10 μg) were treated with RNase-free DNase I and subjected to RT-PCR in the logarithmic phase of amplification (8) using oligonucleotides 2 Pc and Pc for monitoring mat2Pc transcription, oligonucleotides 3M and Mi (Fig. 1) for monitoring mat3Mi transcription, and oligonucleotides polαas and polαs for monitoring polα transcription. The products were resolved by agarose gel electrophoresis and subjected to Southern blotting and hybridization with homologous probes generated by PCR from the plasmid clones as described in Materials and Methods.
FIG. 4
The _sng1_− mutation leads to constitutive expression of mat1Mi transcripts. Primer extension analysis for transcripts Pc (529 nucleotides [nts]) from mat1P and Mi (315 nucleotides) from mat1M, which specifically arise from the mat1 locus. Pc and Mi are indicated by arrows. RNA was isolated from cells of the indicated strains grown in N+ (lanes 1, 3, 5, and 7) and N− (lanes 2, 4, 6 and 8) media at 30°C and subjected to primer extension analysis as described previously (65), using oligonucleotide 1, which is able to reverse transcribe from both mat1Pc and mat1Mi RNAs, as these are known to extend beyond the H2 box (Fig. 1) (31).
FIG. 5
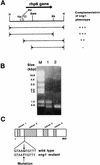
The _sng1_− mutant is encoded by the rhp6+ gene. (A) Complementation of the _sng1_− genotype was tested by subcloning different fragments, as indicated, into pIRT1, transforming them into the _h90 sng1_− mutant strain, and checking for iodine staining and restoration of the temperature-sensitive phenotype. Restriction sites: H, _Hin_dIII; Hp, _Hpa_I; RV, _Eco_RV; Cl, _Cla_I; Bam, _Bam_HI; Xb, _Xba_I. (B) RT-PCR products of RNA for wild-type and mutant strains resolved by agarose gel electrophoresis. Lane M, molecular size markers; lane 1, _rhp6−/sng1_− mutant; lane 2, wild type. (C) Localization of the sng1-1 mutation in the second intron of the rhp6 gene. The gene map showing the intron and exon positions is based on data in reference .
FIG. 6
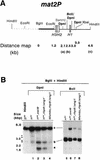
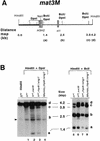
Increased methylase accessibility of the mat2P locus in the _rhp6−/sng1_− mutant is dependent on its utilization for switching. (A) The restriction map of the 6.3-kb _Hin_dIII mat2 fragment shows the distance of various _Sau3_AI sites distal to the _Bgl_II site. (B) Southern blot analysis. Two DNA samples (1 to 2 μg) isolated from strains carrying the chromosomally integrated E. coli dam methylase gene were digested with _Bgl_II plus _Hin_dIII, followed by _Dpn_I (lanes 1 to 4) or _Bcl_I (lanes 5 to 8). The first lane represents the DNA from the h90 strain digested with _Bgl_II plus _Hin_dIII alone. The samples were subjected to electrophoresis; after electrophoresis, the gel was blotted, and the samples were hybridized with the radiolabeled _Bgl_II-_Eco_RI fragment (thick bar in panel A) and subjected to autoradiography. Fragments labeled a, b, and c are defined in panel A. A low level of hybridization was observed consistently due to cross-hybridization elsewhere in the genome, possibly in the centromeric regions as indicated by the arrowheads. One of these cross-hybridizing bands also comigrates with the band at 2.5 kb. The level of methylation of band b was quantitated by subtracting the percent peak area corresponding to the band in the control lane from that determined for lanes 1 to 4 (Table 3). A faint band observed at roughly the position of band a in the _Bcl_I digests (lanes 5 to 8) may also arise due to cross-hybridization with centromeric regions, since there is no _Bcl_I site at the position of band a (26).
FIG. 7
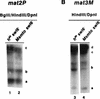
Increased methylase accessibility of the mat3M locus in the _rhp6−/sng1_− mutant also depends on the switching competence. (A) The restriction map of the 4.2-kb _Hin_dIII mat3M DNA fragment shows the distances of different _Bcl_I and _Dpn_I sites from the _Hin_dIII site. (B) Southern blot analysis. DNA samples (1 to 2 μg) isolated from strains carrying the chromosomally integrated E. coli dam methylase gene were digested sequentially with _Hin_dIII plus _Dpn_I (lanes 1 to 4) and _Hin_dIII plus _Bcl_I (lanes 5 to 8). The first lane represents the DNA from the h90 strain digested with _Hin_dIII alone. The samples were subjected to electrophoresis; after electrophoresis, the gel was processed for blotting and hybridization with the _Nsi_I _Hin_dIII fragment, indicated by the thick bar in panel A. The three bands corresponding to cleavage at the _Bcl_I sites are labeled a, b, and c; the uncut 4.2-kb _Hin_dIII band is labeled d. A common band (arrowhead in panel for _Dpn_I digestions) arises from cross-hybridization with sequences elsewhere in the genome, possibly in the centromeric regions, and was not considered for quantitation. The arrow indicates the presence of an additional polymorphic _Sau_3AI site present in our strains but not found in published sequence. This band was also quantitated by densitometry and combined along with band b in Table 4. The centromere-proximal sites are also indicated on the left side of the map in panel A.
FIG. 8
The methylation accessibility of donor loci in the _swi6_− mutant is independent of switching. DNA samples from _h90 swi6_− (lanes 1 and 3) and _Msmto swi6_− (lanes 2 and 4) mutants were digested with _Bgl_II, _Hin_dIII, and _Dpn_I (A, lanes 1 and 2) or _Hin_dIII plus _Dpn_I (B, lanes 3 and 4) and subjected to electrophoresis and Southern hybridization for mat2P (A) and mat3M (B) as described in the legends to Fig. 6 and 7, respectively.
FIG. 9
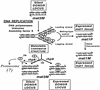
A hypothetical model for the role of rhp6 protein in chromatin assembly. The putative chromatin assembly protein (X), which is transiently expressed during cell cycle and associates with the newly assembled nucleosomes at the replication fork, may help in regulating the proper assembly of an inactive structure, for which its timely removal through ubiquitination by rhp6 and subsequent degradation is very critical. Alternatively, the ubiquitinated (Ub) protein X may remain associated with freshly assembling nucleosomes and play a structural role. In the absence of direct evidence for such a function in this system, ? indicates this possibility as an alternative to that of proteolysis by the proteasome. Nucleosomes of the freshly replicated daughter chromatids in the middle are shaded differently from the parental and the finally assembled inactive daughter chromatids and represent their perturbed state (metastable or potentially active). A close coordination of silencing with switching is indicated by depicting the transposition of the replicated mating-type donor locus to the mat1 locus.
Similar articles
- The fission yeast ubiquitin-conjugating enzymes UbcP3, Ubc15, and Rhp6 affect transcriptional silencing of the mating-type region.
Nielsen IS, Nielsen O, Murray JM, Thon G. Nielsen IS, et al. Eukaryot Cell. 2002 Aug;1(4):613-25. doi: 10.1128/EC.1.4.613-625.2002. Eukaryot Cell. 2002. PMID: 12456009 Free PMC article. - Transcriptional silencing in Saccharomyces cerevisiae and Schizosaccharomyces pombe.
Huang Y. Huang Y. Nucleic Acids Res. 2002 Apr 1;30(7):1465-82. doi: 10.1093/nar/30.7.1465. Nucleic Acids Res. 2002. PMID: 11917007 Free PMC article. Review. - Identification of Uhp1, a ubiquitinated histone-like protein, as a target/mediator of Rhp6 in mating-type silencing in fission yeast.
Naresh A, Saini S, Singh J. Naresh A, et al. J Biol Chem. 2003 Mar 14;278(11):9185-94. doi: 10.1074/jbc.M212732200. Epub 2003 Jan 2. J Biol Chem. 2003. PMID: 12511578 - The rhp6+ gene of Schizosaccharomyces pombe: a structural and functional homolog of the RAD6 gene from the distantly related yeast Saccharomyces cerevisiae.
Reynolds P, Koken MH, Hoeijmakers JH, Prakash S, Prakash L. Reynolds P, et al. EMBO J. 1990 May;9(5):1423-30. doi: 10.1002/j.1460-2075.1990.tb08258.x. EMBO J. 1990. PMID: 2184030 Free PMC article. - Mating-type switching by homology-directed recombinational repair: a matter of choice.
Thon G, Maki T, Haber JE, Iwasaki H. Thon G, et al. Curr Genet. 2019 Apr;65(2):351-362. doi: 10.1007/s00294-018-0900-2. Epub 2018 Oct 31. Curr Genet. 2019. PMID: 30382337 Free PMC article. Review.
Cited by
- Yeast mismatch repair components are required for stable inheritance of gene silencing.
Liu Q, Zhu X, Lindström M, Shi Y, Zheng J, Hao X, Gustafsson CM, Liu B. Liu Q, et al. PLoS Genet. 2020 May 29;16(5):e1008798. doi: 10.1371/journal.pgen.1008798. eCollection 2020 May. PLoS Genet. 2020. PMID: 32469861 Free PMC article. - The fission yeast ubiquitin-conjugating enzymes UbcP3, Ubc15, and Rhp6 affect transcriptional silencing of the mating-type region.
Nielsen IS, Nielsen O, Murray JM, Thon G. Nielsen IS, et al. Eukaryot Cell. 2002 Aug;1(4):613-25. doi: 10.1128/EC.1.4.613-625.2002. Eukaryot Cell. 2002. PMID: 12456009 Free PMC article. - The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe.
Thon G, Hansen KR, Altes SP, Sidhu D, Singh G, Verhein-Hansen J, Bonaduce MJ, Klar AJ. Thon G, et al. Genetics. 2005 Dec;171(4):1583-95. doi: 10.1534/genetics.105.048298. Epub 2005 Sep 12. Genetics. 2005. PMID: 16157682 Free PMC article. - Two ubiquitin-conjugating enzymes, Rhp6 and UbcX, regulate heterochromatin silencing in Schizosaccharomyces pombe.
Choi ES, Kim HS, Jang YK, Hong SH, Park SD. Choi ES, et al. Mol Cell Biol. 2002 Dec;22(23):8366-74. doi: 10.1128/MCB.22.23.8366-8374.2002. Mol Cell Biol. 2002. PMID: 12417737 Free PMC article. - Transcriptional silencing in Saccharomyces cerevisiae and Schizosaccharomyces pombe.
Huang Y. Huang Y. Nucleic Acids Res. 2002 Apr 1;30(7):1465-82. doi: 10.1093/nar/30.7.1465. Nucleic Acids Res. 2002. PMID: 11917007 Free PMC article. Review.
References
- Allshire R C, Nimmo E R, Ekwall K, Javerzat J P, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt centromeric segregation. Genes Dev. 1995;9:218–233. - PubMed
- Anteguera F, Tamame P, Villanueva J, Santos T. DNA methylation in fungi. J Biol Chem. 1984;259:8033–8036. - PubMed
- Bailly V, Lamb J, Sung P, Prakash S, Prakash L. Specific complex formation between yeast RAD6 and RAD18 proteins: a potential mechanism for targeting RAD6 ubiquitin conjugating activity to DNA damage sites. Genes Dev. 1994;8:811–820. - PubMed
- Beach D. Cell type switching by DNA transposition on fission yeast. Nature. 1983;305:682–688.
- Bell S P, Kobayashi R, Stillman B. Yeast origin recognition complex functions in transcriptional silencing and DNA replication. Science. 1993;262:1844–1849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases