Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons - PubMed (original) (raw)
Comparative Study
Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons
P G Mermelstein et al. J Neurosci. 1998.
Abstract
Inwardly rectifying K+ (IRK) channels are critical for shaping cell excitability. Whole-cell patch-clamp and single-cell RT-PCR techniques were used to characterize the inwardly rectifying K+ currents found in projection neurons of the rat nucleus accumbens. Inwardly rectifying currents were highly selective for K+ and blocked by low millimolar concentrations of Cs+ or Ba2+. In a subset of neurons, the inwardly rectifying current appeared to inactivate at hyperpolarized membrane potentials. In an attempt to identify this subset, neurons were profiled using single-cell RT-PCR. Neurons expressing substance P mRNA exhibited noninactivating inward rectifier currents, whereas neurons expressing enkephalin mRNA exhibited inactivating inward rectifier currents. The inactivation of the inward rectifier was correlated with the expression of IRK1 mRNA. These results demonstrate a clear physiological difference in the properties of medium spiny neurons and suggest that this difference could influence active state transitions driven by cortical and hippocampal excitatory input.
Figures
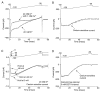
Fig. 1.
Cs+ blocks inwardly rectifying K+ currents in NAcc neurons. A, A series of voltage steps (−30 to −120 mV in 10 mV increments) from −50 mV (E_K) produces an inwardly rectifying K+ current. The asterisk indicates the beginning of outward rectification at −30 mV, predicted by the_I–V relationship of voltage-gated K+currents. B, Addition of 1 m
m
Cs+ to the extracellular recording solution preferentially eliminated the inward component of the current.C, The Cs+-sensitive current (subtraction of the traces in A from B) isolates the inwardly rectifying K+ current.D, Current–voltage relationship of the inwardly rectifying K+ current (measured at the_arrow_ in C).
Fig. 2.
Holding at or below −50 mV minimizes deactivating tail currents. A, A depolarizing voltage ramp to −30 mV after a brief (16 msec) step to −120 mV from a holding potential of −50 mV produces an inwardly rectifying K+ current. The current is sensitive to 1 m
m
Cs+.B, Subtraction of the traces in _A_isolates the inward rectifier. The Cs+-sensitive current when using ramp protocols is comparable to the current measured with steps (the currents observed with a series of voltage steps are displayed as overlaid circles). C, Holding at either −50 or −80 mV produces a similar inwardly rectifying current, whereas holding at 0 mV produces a tail current. Similar results were seen in three other cells. The data suggest that at −80 and −50 mV, voltage-gated K+ currents are not contributing to the inward current. D, The inwardly rectifying current is observed after removal of extracellular Ca2+ and the addition of a intracellular calcium chealator (5 m
m
EGTA), suggesting Ca2+-dependent K+ currents are also not responsible for this current. Similar results were seen in five other cells.
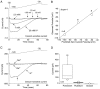
Fig. 3.
The inwardly rectifying current is K+-selective. A, Decreasing extracellular K+ shifts the zero current potential of the inward rectifier in a manner consistent with a K+-selective channel. B, Summarized data in which the predicted zero current potential is compared with the observed potential (n ≥ 6; mean ± SEM; error bars are smaller than the circles). C, Substitution of extracellular K+ with either Rb+ or Na+ drastically attenuated the inward current. D, Box plot summary of the inward current (measured at the time indicated by the arrow in_C_) in the presence of 20 m
m
K+, Rb+, or Na+ (n = 6). For selectivity estimates,_I_Rb/_I_K = 0.07, whereas_I_Na/_I_K = 0.001, indicating a current highly selective for K+.
Fig. 4.
The block of the inward rectifier by Cs+ is dose-dependent. A, Increasing concentrations of Cs+ from 10 μ
m
to 10 m
m
increased the block of the inward rectifier.B, Subtraction of the traces in A isolate the inward rectifier. Maximal block was seen with low millimolar concentrations of Cs+. Similar results were seen with Ba2+ (n = 4; data not shown). C, Summarized dose–response data for Cs+ block (n = 7; mean ± SEM). The _IC_50 was 32 μ
m
.Inset, Hill plot of Cs+ block. Error bars (±SEM) are smaller than the circles. The slope was slightly <1 (0.8).
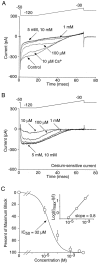
Fig. 5.
The inwardly rectifying K+current inactivates in a subpopulation of NAcc neurons.A, In many neurons, a hyperpolarizing step to −120 mV resulted in an inward current that displayed little inactivation.B, However, in a subpopulation of cells (∼40%), a significant component of the inwardly rectifying K+current inactivated. C, The inactivation cannot be attributed to blockade of IRK channels by extracellular monovalent cations other than K+, because inactivation was still observed when these were replaced with sucrose. Similar results were seen in five other cells. D, The ERG potassium blocker terfenadine (3 μ
m
) had no effect on the inactivating inward current, demonstrating the IRK recordings were not contaminated with other potassium channels. Inset, Box plot summary of the teranadine effect in 12 neurons. Terfenadine blocked 3.5 ± 1.1% of the whole-cell inward current.
Fig. 6.
Inactivation of the inward rectifier is dependent on voltage and not _E_K. A, Shifting _E_K had no effect on inactivation. Comparison of the inward current in which _E_Kwas either −50 or −75 mV. B, Adjusting for differences in driving force, the lack of an effect of_E_K is more easily resolved. Similar results were observed in four other cells. C, Inactivation of the inward rectifier was voltage-dependent. Although the inward current inactivates at both −90 and −120 mV, stronger hyperpolarizations produced more rapid inactivation kinetics (n = 8). Because the onset of Cs+ block is voltage-dependent (see Results for details) non-Cs+-subtracted traces are provided. Inset, The Cs+-sensitive current at −120 mV is provided for comparative purposes. D, Adjusting for driving force, the differences in inactivation kinetics between voltages is more apparent. E, Recovery from inactivation was rapid, occurring with progressively longer depolarizations to −50 mV.F, In this example neuron, the time of recovery could be fit with a single exponential with a tau equaling 75 msec. Similar results were seen in three other neurons.
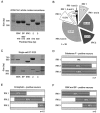
Fig. 7.
IRK and peptide mRNA expression are correlated in individual NAcc neurons. A, IRK1–3 PCR products are detected using cDNA from whole NAcc tissue. ENK and SP peptide products are also observed. B, IRK1–3 mRNA expression in individual neurons varied (n = 39). The most common phenotype expressed IRK2 and IRK3 (n = 13 of 39). Notice the low percentage of cells displaying detectable levels of all three subunit mRNAs (n = 3 of 39).C, Single cells showing the correlation of IRK subunits with peptide expression. The ENK mRNA-positive neuron (top) expressed detectable levels of IRK1 and IRK2. On the other hand, the SP-positive neuron (bottom) expressed IRK2 and IRK3. D_–_F, Summarized data comparing IRK mRNA subunit expression in SP-positive (n = 7; D) and ENK-positive (n = 15; E) neurons and neurons expressing both ENK and SP (n = 5;F). Coexpression of multiple IRK mRNA subunits can be deduced by the extent of bar overlap. NAcc neurons typically expressed IRK2, regardless of peptide expression. However, IRK1 expression was only found in ENK-positive neurons, although IRK3 expression was most often present in SP-positive cells.
Fig. 8.
The inactivating, inwardly rectifying K+ current is correlated with IRK1 mRNA expression.A, An enkephalin- and substance P-positive neuron in which a large proportion of the inwardly rectifying current inactivated. B, Another neuron in which the inward rectifier did not inactivate. This cell expressed substance P alone.C, Box plot summary comparing the proportion of the whole-cell current that inactivated in different NAcc neurons. Neurons expressing ENK alone (n = 8) displayed more inactivation than neurons only expressing SP (n = 5) but less than cells expressing both ENK and SP (n = 4). D, Comparison between different NAcc neurons (based on peptide expression) and their probability of expressing detectable levels of IRK1. Peptide expression was correlated with both IRK1 mRNA expression and the amount of current inactivation.
Similar articles
- HERG- and IRK-like inward rectifier currents are sequentially expressed during neuronal development of neural crest cells and their derivatives.
Arcangeli A, Rosati B, Cherubini A, Crociani O, Fontana L, Ziller C, Wanke E, Olivotto M. Arcangeli A, et al. Eur J Neurosci. 1997 Dec;9(12):2596-604. doi: 10.1111/j.1460-9568.1997.tb01689.x. Eur J Neurosci. 1997. PMID: 9517465 - Dopamine D(2) receptor modulation of K(+) channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials.
Perez MF, White FJ, Hu XT. Perez MF, et al. J Neurophysiol. 2006 Nov;96(5):2217-28. doi: 10.1152/jn.00254.2006. Epub 2006 Aug 2. J Neurophysiol. 2006. PMID: 16885524 - Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells.
Takigawa T, Alzheimer C. Takigawa T, et al. J Physiol. 2002 Feb 15;539(Pt 1):67-75. doi: 10.1113/jphysiol.2001.012883. J Physiol. 2002. PMID: 11850502 Free PMC article. - Analysis of G-protein-activated inward rectifying K(+) (GIRK) channel currents upon GABAB receptor activation in rat supraoptic neurons.
Harayama N, Kayano T, Moriya T, Kitamura N, Shibuya I, Tanaka-Yamamoto K, Uezono Y, Ueta Y, Sata T. Harayama N, et al. Brain Res. 2014 Dec 3;1591:1-13. doi: 10.1016/j.brainres.2014.10.022. Epub 2014 Oct 23. Brain Res. 2014. PMID: 25451091
Cited by
- Blinded prospective evaluation of computer-based mechanistic schizophrenia disease model for predicting drug response.
Geerts H, Spiros A, Roberts P, Twyman R, Alphs L, Grace AA. Geerts H, et al. PLoS One. 2012;7(12):e49732. doi: 10.1371/journal.pone.0049732. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251349 Free PMC article. - Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors.
Galarraga E, Hernández-López S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C, Bargas J. Galarraga E, et al. J Neurosci. 1999 May 1;19(9):3629-38. doi: 10.1523/JNEUROSCI.19-09-03629.1999. J Neurosci. 1999. PMID: 10212321 Free PMC article. - Eating "junk food" has opposite effects on intrinsic excitability of nucleus accumbens core neurons in obesity-susceptible versus -resistant rats.
Oginsky MF, Ferrario CR. Oginsky MF, et al. J Neurophysiol. 2019 Sep 1;122(3):1264-1273. doi: 10.1152/jn.00361.2019. Epub 2019 Jul 31. J Neurophysiol. 2019. PMID: 31365322 Free PMC article. - Altered enhancer transcription underlies Huntington's disease striatal transcriptional signature.
Le Gras S, Keime C, Anthony A, Lotz C, De Longprez L, Brouillet E, Cassel JC, Boutillier AL, Merienne K. Le Gras S, et al. Sci Rep. 2017 Feb 22;7:42875. doi: 10.1038/srep42875. Sci Rep. 2017. PMID: 28225006 Free PMC article. - A cholinergic-regulated circuit coordinates the maintenance and bi-stable states of a sensory-motor behavior during Caenorhabditis elegans male copulation.
Liu Y, LeBeouf B, Guo X, Correa PA, Gualberto DG, Lints R, Garcia LR. Liu Y, et al. PLoS Genet. 2011 Mar;7(3):e1001326. doi: 10.1371/journal.pgen.1001326. Epub 2011 Mar 10. PLoS Genet. 2011. PMID: 21423722 Free PMC article.
References
- Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels. 1994;2:183–191. - PubMed
- Chronister RB, DeFrance JF. Nucleus accumbens in historical perspective. In: Chronister RB, DeFrance JF, editors. The neurobiology of the nucleus accumbens. Haer Institute for Electrophysiological Research; Brunswick, ME: 1981. pp. 1–6.
- Demo SD, Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991;7:743–753. - PubMed
- Doupnik CA, Davidson N, Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995;5:268–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034696/NS/NINDS NIH HHS/United States
- P01 MH040899/MH/NIMH NIH HHS/United States
- F32 NS010028/NS/NINDS NIH HHS/United States
- MH-40899/MH/NIMH NIH HHS/United States
- R37 NS034696/NS/NINDS NIH HHS/United States
- NS-34696/NS/NINDS NIH HHS/United States
- NS-10028/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources