Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons - PubMed (original) (raw)
Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons
A Araque et al. J Neurosci. 1998.
Abstract
Astrocytes exhibit a form of excitability and communication on the basis of intracellular Ca2+ variations (Cornell-Bell et al., 1990; Charles et al., 1991) that can be initiated by neuronal activity (Dani et al., 1992; Porter and McCarthy, 1996). A Ca2+ elevation in astrocytes induces the release of glutamate (Parpura et al., 1994; Pasti et al., 1997; Araque et al., 1998;Bezzi et al., 1998), which evokes a slow inward current in neurons and modulates action potential-evoked synaptic transmission between cultured hippocampal cells (Araque et al., 1998), suggesting that astrocytes and neurons may function as a network with bidirectional communication. Here we show that a Ca2+ elevation in astrocytes increases the frequency of excitatory as well as inhibitory miniature postsynaptic currents (mPSCs), without modifying their amplitudes. Thapsigargin incubation, microinjection of the Ca2+ chelator BAPTA, and photolysis of the Ca2+ cage NP-EGTA demonstrate that a Ca2+ elevation in astrocytes is both necessary and sufficient to modulate spontaneous transmitter release. This Ca2+-dependent release of glutamate from astrocytes enhances mPSC frequency by acting on NMDA glutamate receptors, because it is antagonized by D-2-amino-5-phosphonopentanoic acid (AP5) or extracellular Mg2+. These NMDA receptors are located extrasynaptically, because blockage specifically of synaptic NMDA receptors by synaptic activation in the presence of the open channel blocker MK-801 did not impair the AP5-sensitive astrocyte-induced increase of mPSC frequency. Therefore, astrocytes modulate spontaneous excitatory and inhibitory synaptic transmission by increasing the probability of transmitter release via the activation of NMDA receptors.
Figures
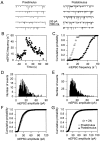
Fig. 1.
Astrocyte stimulation increases mEPSC frequency. A, mEPSCs recorded at a holding potential of −60 mV before and after mechanical stimulation of an astrocyte.B, Time course of the mEPSC frequency calculated in 1 sec bins. Zero time corresponds to the time of astrocyte stimulation.C, Cumulative probability plot of the mEPSC frequency 30 sec before and after astrocyte stimulation (open and_filled symbols_, respectively). D,E, Histograms of mEPSC amplitudes (bin width, 1 pA) recorded 30 sec before and after astrocyte stimulation, respectively.F, Cumulative probability plot of the mEPSC amplitudes recorded 30 sec before and after astrocyte stimulation (open and filled symbols, respectively).G, Average (n = 24) cumulative probability plot of the mEPSC amplitudes recorded 30 sec before and after astrocyte stimulation (open and filled symbols, respectively). Error bars showing SEM are smaller than symbol size. To obtain cumulative probability plots, we calculated the frequency and the amplitudes of mEPSCs in 1 sec and 1 pA bins, respectively.
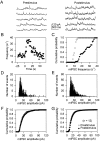
Fig. 2.
Astrocyte stimulation increases mIPSC frequency.A, mIPSCs recorded at a holding potential of −30 mV before and after mechanical stimulation of an astrocyte.B, Time course of the mIPSC frequency calculated in 1 sec bins. Zero time corresponds to the time of astrocyte stimulation.C, Cumulative probability plot of the mIPSC frequency 30 sec before and after astrocyte stimulation (open and_filled symbols_, respectively). D,E, Histograms of mIPSC amplitudes recorded 30 sec before and after astrocyte stimulation, respectively. F, Cumulative probability plot of the mIPSC amplitudes recorded 30 sec before and after astrocyte stimulation (open and_filled symbols_, respectively). G, Average (n = 12) cumulative probability plot of the mEPSC amplitudes recorded 30 sec before and after astrocyte stimulation (open and filled symbols, respectively). Cumulative probability plots were obtained as in Figure 1.
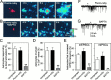
Fig. 3.
Microinjection of the Ca2+chelator BAPTA into an astrocyte prevents the propagation of astrocyte Ca2+ waves and blocks the astrocyte-induced increase in mPSC frequency. A, Cultures were loaded with the Ca2+ indicator fluo-3 to monitor the stimulus-induced Ca2+ elevations in astrocytes, and a single astrocyte was microinjected with fluoro-ruby (left panel). Right panels show images in pseudocolor mode representing intensity of fluo-3 emission taken before, during, and after mechanical stimulation of the fluoro-ruby-injected cell at the times indicated. Zero time corresponds to the time of astrocyte stimulation. Mechanical stimulation increases intracellular Ca2+ in the injected cell as well as in neighboring unstimulated astrocytes. B, Same as in_A_ but with a single astrocyte microinjected with fluoro-ruby and BAPTA (left panel). Mechanical stimulation of the injected cell did not change the fluorescent emission of fluo-3 either in the stimulated or neighboring astrocytes.C, D, Quantitative data taken from these experiments. The number of astrocytes involved in Ca2+ waves was quantified by the proportion of nonstimulated cells within the field of view that responded with a Ca2+ elevation. Although the ability of astrocytes to respond to direct stimulation or to evoke Ca2+waves (C and D, respectively) was unaffected by the injection of fluoro-ruby, it was reduced significantly by BAPTA injection. In parallel studies mPSCs were recorded in response to mechanical stimulation of astrocytes. Whereas the astrocyte-induced mPSC frequency increase was not affected by the injection of fluoro-ruby (E, F), it was prevented by the injection of BAPTA (E,G). **p < 0.01.
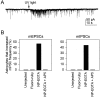
Fig. 4.
Ca2+ elevation in astrocytes is sufficient to increase the frequency of mPSCs. A, Whole-cell recording from a neuron adjacent to an astrocyte that had been microinjected with the UV-sensitive Ca2+ cage NP-EGTA. UV photolysis (arrow) increased the Ca2+ level in the astrocyte and caused an increase in the frequency of mEPSCs. B, Graphs summarizing the effects of photolysis on the mEPSC and mIPSC frequency. Pulses of UV light only increased the frequency of mPSCS when the astrocyte was injected with NP-EGTA. Although the frequency of mPSCs was not modified by UV stimulation of uninjected astrocytes (0 of 6 cells) or astrocytes injected with fluoro-ruby alone (0 of 22 cells), photolysis of NP-EGTA-injected astrocytes increased the frequency of mEPSCs (8 of 17 cells) and mIPSCs (4 of 9 cells). This photolysis-dependent increase in mPSC frequency was prevented by incubation with 50 μ
m
AP5 (0 of 12 NP-EGTA-injected astrocytes).
Fig. 5.
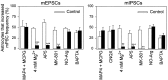
Astrocyte-induced increase in mPSC frequency is mediated by NMDA receptors. Shown is the percentage of mechanically stimulated astrocytes that increased the frequency of mEPSCs (left) and mIPSCs (right) in 0.5 m
m
MAP4 and 0.5 m
m
MCPG, 10 μ
m
CNQX, 4 m
m
Mg2+, 50 μ
m
AP5, and 50 μ
m
NO-Arg; also shown is the percentage after dialysis of the postsynaptic cell with BAPTA (10 m
m
in the recording pipette) and in their respective control solution in parallel cultures. In the histograms labeled MK-801 we caused a use-dependent block of NMDA receptors before stimulating astrocytes by incubating the cultures during 5 min in NMDA (200 μ
m
) and MK-801 (5 μ
m
) to cause a sustained block of this receptor subtype (see Results). After washout, astrocyte stimulation no longer modulated mPSC frequency. Significant differences with respect to control were established by the Student’s t test at **p < 0.01; ***p < 0.001.
Fig. 6.
AP5-sensitive astrocyte-induced increase in mPSC frequency is not mediated by synaptic NMDA receptors.A, mPSCs recorded at a holding potential of −60 mV in control solution (left). To block postsynaptic NMDA receptors, we added MK-801 to the saline while the synaptic release of glutamate was stimulated by the pressure ejection of high osmolarity saline (center). Several 1- to 50-sec-duration pressure pulses of high osmolarity solution (obtained by the addition of 0.3
m
sucrose to the standard saline) were delivered, but only one is shown. Subsequently, MK-801 was washed out of the saline.Right, mPSCs recorded at a holding potential of −30 mV after the blockage of postsynaptic NMDA receptors with MK-801. The_trace_ has been offset for illustration purposes. Mechanical stimulation of the astrocyte is indicated by the_asterisk_. Note that, despite the selective block of synaptic NMDA receptors, the stimulation of astrocytes still evoked an increase in the frequency of mPSCs. B, Averaged (n > 50) mEPSCs (dotted lines) in control solution and in MK-801 after several pressure pulses of high osmolarity solution. Synaptic activation with high osmolarity saline blocked postsynaptic NMDA receptors, because mEPSCs now exhibited only one time constant of decay. The decay time course of mEPSCs was fit to two and to a single exponential function in control and after MK-801 treatment, respectively (continuous lines).C, Proportion of astrocytes in which mechanical stimulation evoked an increase in the frequency of mEPSCs (left) and mIPSCs (right) in control solution and after the blockage of synaptic NMDA receptors with MK-801 and high osmolarity saline in the absence and in the presence of 50 μ
m
AP5. **p < 0.01.
Similar articles
- Astrocytic control of synaptic NMDA receptors.
Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Lee CJ, et al. J Physiol. 2007 Jun 15;581(Pt 3):1057-81. doi: 10.1113/jphysiol.2007.130377. Epub 2007 Apr 5. J Physiol. 2007. PMID: 17412766 Free PMC article. - Mechanisms underlying the enhancement of excitatory synaptic transmission in basolateral amygdala neurons of the kindling rat.
Shoji Y, Tanaka E, Yamamoto S, Maeda H, Higashi H. Shoji Y, et al. J Neurophysiol. 1998 Aug;80(2):638-46. doi: 10.1152/jn.1998.80.2.638. J Neurophysiol. 1998. PMID: 9705457 - Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons.
Araque A, Parpura V, Sanzgiri RP, Haydon PG. Araque A, et al. Eur J Neurosci. 1998 Jun;10(6):2129-42. doi: 10.1046/j.1460-9568.1998.00221.x. Eur J Neurosci. 1998. PMID: 9753099 - Astrocyte-induced modulation of synaptic transmission.
Araque A, Sanzgiri RP, Parpura V, Haydon PG. Araque A, et al. Can J Physiol Pharmacol. 1999 Sep;77(9):699-706. Can J Physiol Pharmacol. 1999. PMID: 10566947 Review. - Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP.
Fellin T, Sul JY, D'Ascenzo M, Takano H, Pascual O, Haydon PG. Fellin T, et al. Novartis Found Symp. 2006;276:208-17; discussion 217-21, 233-7, 275-81. doi: 10.1002/9780470032244.ch16. Novartis Found Symp. 2006. PMID: 16805432 Review.
Cited by
- Role of calcium in neurotensin-evoked enhancement in firing in mesencephalic dopamine neurons.
St-Gelais F, Legault M, Bourque MJ, Rompré PP, Trudeau LE. St-Gelais F, et al. J Neurosci. 2004 Mar 10;24(10):2566-74. doi: 10.1523/JNEUROSCI.5376-03.2004. J Neurosci. 2004. PMID: 15014132 Free PMC article. - Challenges and opportunities of advanced gliomodulation technologies for excitation-inhibition balance of brain networks.
Chen K, Stieger KC, Kozai TD. Chen K, et al. Curr Opin Biotechnol. 2021 Dec;72:112-120. doi: 10.1016/j.copbio.2021.10.008. Epub 2021 Nov 10. Curr Opin Biotechnol. 2021. PMID: 34773740 Free PMC article. Review. - The astrocyte odyssey.
Wang DD, Bordey A. Wang DD, et al. Prog Neurobiol. 2008 Dec 11;86(4):342-67. doi: 10.1016/j.pneurobio.2008.09.015. Epub 2008 Oct 1. Prog Neurobiol. 2008. PMID: 18948166 Free PMC article. Review. - Astrocytes optimize the synaptic transmission of information.
Nadkarni S, Jung P, Levine H. Nadkarni S, et al. PLoS Comput Biol. 2008 May 30;4(5):e1000088. doi: 10.1371/journal.pcbi.1000088. PLoS Comput Biol. 2008. PMID: 18516277 Free PMC article. - Physiology of Astroglia.
Verkhratsky A, Nedergaard M. Verkhratsky A, et al. Physiol Rev. 2018 Jan 1;98(1):239-389. doi: 10.1152/physrev.00042.2016. Physiol Rev. 2018. PMID: 29351512 Free PMC article. Review.
References
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. - PubMed
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are colocalized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi Rizzini B, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous