Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus - PubMed (original) (raw)
Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus
W Gottschalk et al. J Neurosci. 1998.
Abstract
In addition to the regulation of neuronal survival and differentiation, neurotrophins may play a role in synapse development and plasticity. Application of brain-derived neurotrophic factor (BDNF) promotes long-term potentiation (LTP) in CA1 synapses of neonatal hippocampus, which otherwise exhibit only short-term potentiation. This is attributable, at least in part, to an attenuation of the synaptic fatigue induced by high-frequency stimulation (HFS). However, the prevention of synaptic fatigue by BDNF could be mediated by an attenuation of synaptic vesicle depletion from presynaptic terminals and/or a reduction of the desensitization of postsynaptic receptors. Here we provide evidence supporting a presynaptic effect of BDNF. The effect of BDNF on synaptic fatigue depended on the stimulation frequency, not on the stimulus duration nor on the number of stimulation pulses. BDNF was only effective when the synapses were stimulated at frequencies >50 Hz. Treatment with BDNF also potentiated paired-pulse facilitation (PPF), a parameter reflecting changes in the properties of presynaptic terminals. This effect of BDNF was restricted only to PPF elicited with interpulse intervals </=20 msec. Changes in the extracellular calcium concentration altered the magnitude of the BDNF effect on PPF and synaptic responses to HFS, suggesting that BDNF regulates neurotransmitter release. When the desensitization of glutamate receptors was blocked by cyclothiazide or aniracetam, the BDNF potentiation of the synaptic responses to HFS was unaltered. Taken together, these results suggest that BDNF acts presynaptically. When two pathways in the same slice were monitored simultaneously, BDNF treatment potentiated the tetanized pathway without affecting the synaptic efficacy of the untetanized pathway. The selective potentiation of high-frequency transmission by BDNF appears to contribute directly to the effect of BDNF on LTP rather than indirectly by inducing the release of additional diffusible factors. The preferential potentiation of highly active synapses by BDNF may have implications in the Hebbian mechanism of synaptic plasticity.
Figures
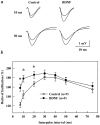
Fig. 1.
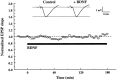
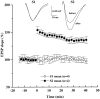
BDNF does not affect basal synaptic transmission in CA1 synapses of P12–P13 hippocampus. The slopes of the EPSPs were measured every 30 sec before and during continuous application of BDNF to the interface chamber (black bar, up to 3 hr). Each_point_ represents the average slope of 10 consecutive EPSPs (5 min interval) normalized to the average slope of the EPSPs acquired before BDNF application (20 min baseline;n = 5). Representative traces acquired before and after 3 hr of BDNF treatment are shown at the top(averages of 10 consecutive traces). In this and all of the following figures, slices from P12–P13 rats were used. BDNF was applied to the interface chamber at the final concentration of 2 n
m
for 3 hr.
Fig. 2.
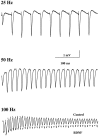
Synaptic responses to repetitive stimulation at different frequencies. Shown are representative field EPSPs elicited by trains of afferent stimulation at the indicated frequencies. Because no difference was seen between control and BDNF-treated slices of EPSPs elicited by 25 and 50 Hz stimulation, only control traces are presented. At 100 Hz, EPSPs from BDNF-treated slices are superimposed on those from control slices.
Fig. 3.
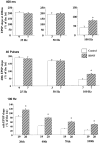
Effect of BDNF on synaptic responses to repetitive afferent stimulation is dependent on the stimulation frequency. Repetitive stimulation was delivered to afferent fibers. One of three variables (duration, number of pulses, or stimulation frequency) was held constant. The last EPSP slopes at different conditions were calculated as the percentage of the first EPSP slope. The data were analyzed by ANOVA, followed by Fisher’s post hoc test (*p < 0.05). In this and all of the other figures, the numbers associated with each _column_represent the number of pathways recorded. a, BDNF effect on EPSPs elicited by stimulation at different frequencies with a fixed train duration (400 msec). BDNF affected only responses to stimulation at 100 Hz. b, BDNF effect on EPSPs elicited by stimulation at different frequencies with a fixed number of pulses (40 pulses). Only those elicited by 100 Hz stimulation exhibited a difference between control and BDNF-treated slices. c, BDNF effect on EPSPs elicited by stimulation with different numbers of pulses at a fixed frequency (100 Hz). Data on the 20th, 40th, 70th, and 100th pulse are shown. In all cases the EPSP slopes are higher in BDNF-treated slices as compared with the control.
Fig. 4.
Effect of BDNF on fiber volley. To better visualize the fiber volley elicited by high-frequency stimulation, we treated slices with a low-calcium ACSF ([Ca2+]o = 0.5 m
m
) plus cadmium (0.5 m
m
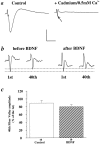
) to block the influx of calcium.a, Representative traces of EPSPs before (left) and after (right) the application of cadmium/0.5 m
m
[Ca2+]oACSF. Note that this treatment virtually eliminated the EPSP, allowing for the isolation of the fiber volley (arrow), which is masked by the EPSP in the control trace (left).b, Representative traces of the first and 40th fiber volleys during a 100 Hz train of afferent stimulation before (left) and 3 hr after (right) BDNF treatment in the same slice. Vertical scale: 1 mV for a_and 0.5 mV for b; horizontal scale: 100 msec for_a and 70 msec for b. c, Summary of BDNF effect on fiber volley. A train of 100 Hz pulses was delivered to the slices for 1 sec, and the isolated fiber volley was recorded. The amplitude of the 40th fiber volley is presented as a percentage of the first fiber volley in control and BDNF-treated slices. No significant differences were found in the fiber volley amplitudes between control and BDNF-treated slices (Control: 89% ± 6.9, n = 10; BDNF: 80% ± 5.6,n = 10).
Fig. 5.
BDNF enhances paired-pulse facilitation at short interpulse intervals. Each slice was tested for PPF in one pathway at different interpulse intervals from 7 to 200 msec (only intervals 7–75 msec are shown). BDNF was added to the perfusion medium; 3 hr later a second pathway was stimulated with paired stimuli at the same intervals. a, Representative examples of PPF recorded at 10 or 50 msec interpulse intervals before and after BDNF treatment. Traces of the first and second EPSPs are superimposed. Note that, at the 10 msec interval, BDNF significantly increased the size of the second EPSP without affecting the size of the first EPSP.b, PPF ratio curves showing facilitation at different interpulse intervals. BDNF significantly affected PPF only at interpulse intervals between 7 and 20 msec (*p < 0.05, ANOVA).
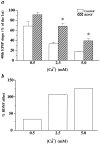
Fig. 6.
The effect of BDNF on synaptic fatigue at different [Ca2+]o. a, BDNF effect on EPSPs elicited by HFS (100 Hz, 1 sec) at different [Ca2+]o. Synaptic responses to HFS under different conditions are measured as a percentage of the 40th EPSP slope over the first EPSP slope. The data were analyzed by ANOVA, followed by Fisher’s post hoc test. Statistically significant differences between a control and BDNF pair at a particular [Ca2+]o are marked by an_asterisk_ (p < 0.05). The_numbers_ associated with each column are as follows. At 0.5 m
m
[Ca2+]o, Control: 68% ± 9.0,n = 3; BDNF: 91% ± 15.3, n = 4. At 2.5 m
m
[Ca2+]o, Control: 33% ± 3.1,n = 3; BDNF: 68% ± 6.1, n = 4. At 5.0 m
m
[Ca2+]o, Control: 17% ± 0.33,n = 3; BDNF: 39% ± 4.1, n = 4. Note that at 0.5 m
m
[Ca2+]o the difference between control and BDNF groups is not statistically significant. b, The percentage of change seen after the addition of BDNF in the various [Ca2+]o during HFS. All calculations are done with data from a. The percentage of BDNF effect under different [Ca2+]o = (BDNF − Control)/Control (0.5 m
m
, 33%; 2.5 m
m
, 106%; 5.0 m
m
, 125%).
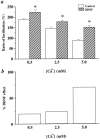
Fig. 7.
The effect of BDNF on PPF at different [Ca2+]o. a, The effect of BDNF on PPF at a 10 msec interpulse interval at different [Ca2+]o. PPF is measured as the ratio of facilitation (%). The data were analyzed by ANOVA, followed by Fisher’s post hoc test. Statistically significant differences between a control and BDNF pair at a particular [Ca2+]o are marked by an_asterisk_ (p < 0.05). The_numbers_ associated with each column are as follows. At 0.5 m
m
[Ca2+]o, Control: 188% ± 4.3,n = 6; BDNF: 224 ± 11.6,n = 10. At 2.5 m
m
[Ca2+]o, Control: 144% ± 8.2,n = 8; BDNF: 178% ± 7.3, n = 8. At 5.0 m
m
[Ca2+]o, Control: 89% ± 4.6,n = 6; BDNF: 151% ± 8.7, n = 12. b, The percentage change of PPF seen after the addition of BDNF in the various [Ca2+]o. All calculations are done with data from a. The percentage of BDNF effect under different [Ca2+]o = (BDNF − Control)/Control (0.5 m
m
, 19%; 2.5 m
m
, 23%; 5.0 m
m
, 70%).
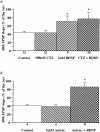
Fig. 8.
Blockade of desensitization of non-NMDA-type glutamate receptors does not alter the BDNF-induced increases in synaptic responses to HFS. Cyclothiazide (a,CTZ, 0.1 m
m
) or aniracetam (b, Anirac., 5 m
m
) was applied to the slices either alone or 30 min before BDNF application. EPSPs evoked by HFS were compared before and 3 hr after BDNF and CTZ or aniracetam application. The slopes of the 40th EPSP are presented as a percentage of the first EPSP slope. The magnitude of the BDNF attenuation of the synaptic fatigue during HFS is unaltered in the presence of CTZ or aniracetam (*p < 0.05, ANOVA).
Fig. 9.
BDNF selectively facilitates the induction of LTP in the tetanized pathway without affecting the synaptic efficacy of the untetanized pathway. EPSPs were recorded in the CA1 area of BDNF-treated slices. Two stimulating electrodes were positioned on either side of a single recording electrode to stimulate two different groups of afferents converging in the same dendritic field in CA1. Stimulation was applied to Schaffer collaterals alternately at low frequency (1 per min). After a period of baseline recording, LTP was induced with a theta burst stimulation applied at time 0_only to one pathway (S1, filled squares). Simultaneous recording of an independent pathway (S2,open circles) showed no change in its synaptic strength after the theta burst was delivered to S1. Synaptic efficacy (initial slope of field EPSPs) is expressed as a percentage of baseline value recorded during the 20 min before the tetanus. Representative traces of field EPSPs from S1 and_S2 pathways were taken 10 min before and 40 min after the theta burst stimulation.
Fig. 10.
Schematic model for the selective potentiation of active synapses by BDNF. High-frequency stimulation elicits synthesis and/or release of BDNF, increasing its concentration surrounding the hippocampal neurons. BDNF may potentiate transmitter release only at synapses that undergo high-frequency transmission (red) without affecting less active synapses (blue).
Similar articles
- Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice.
Abidin I, Köhler T, Weiler E, Zoidl G, Eysel UT, Lessmann V, Mittmann T. Abidin I, et al. Eur J Neurosci. 2006 Dec;24(12):3519-31. doi: 10.1111/j.1460-9568.2006.05242.x. Eur J Neurosci. 2006. PMID: 17229100 - Associative synaptic plasticity in hippocampal CA1 neurons is not sensitive to unpaired presynaptic activity.
Buonomano DV, Merzenich MM. Buonomano DV, et al. J Neurophysiol. 1996 Jul;76(1):631-6. doi: 10.1152/jn.1996.76.1.631. J Neurophysiol. 1996. PMID: 8836251 - Attenuation of paired-pulse facilitation associated with synaptic potentiation mediated by postsynaptic mechanisms.
Wang JH, Kelly PT. Wang JH, et al. J Neurophysiol. 1997 Nov;78(5):2707-16. doi: 10.1152/jn.1997.78.5.2707. J Neurophysiol. 1997. PMID: 9356420 - Neurotrophins and hippocampal synaptic transmission and plasticity.
Lu B, Chow A. Lu B, et al. J Neurosci Res. 1999 Oct 1;58(1):76-87. J Neurosci Res. 1999. PMID: 10491573 Review. - BDNF-induced local protein synthesis and synaptic plasticity.
Leal G, Comprido D, Duarte CB. Leal G, et al. Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
- A treadmill running research protocol to assess dynamic visual acuity and balance for athletes with and without recent concussion history.
Mitchell KM, Dalton KN, Cinelli ME. Mitchell KM, et al. BMC Sports Sci Med Rehabil. 2024 May 17;16(1):112. doi: 10.1186/s13102-024-00900-x. BMC Sports Sci Med Rehabil. 2024. PMID: 38760838 Free PMC article. - A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain.
Jang K, Garraway SM. Jang K, et al. Neurobiol Pain. 2024 Jan 20;15:100151. doi: 10.1016/j.ynpai.2024.100151. eCollection 2024 Jan-Jun. Neurobiol Pain. 2024. PMID: 38314104 Free PMC article. Review. - Gut Microbiota Taxon-Dependent Transformation of Microglial M1/M2 Phenotypes Underlying Mechanisms of Spatial Learning and Memory Impairment after Chronic Methamphetamine Exposure.
Wu Y, Dong Z, Jiang X, Qu L, Zhou W, Sun X, Hou J, Xu H, Cheng M. Wu Y, et al. Microbiol Spectr. 2023 Jun 15;11(3):e0030223. doi: 10.1128/spectrum.00302-23. Epub 2023 May 22. Microbiol Spectr. 2023. PMID: 37212669 Free PMC article. - BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders.
Bazzari AH, Bazzari FH. Bazzari AH, et al. Int J Mol Sci. 2022 Jul 29;23(15):8417. doi: 10.3390/ijms23158417. Int J Mol Sci. 2022. PMID: 35955546 Free PMC article. Review. - Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Mediating Function and Dysfunction at Glutamatergic Synapses.
Mohanan AG, Gunasekaran S, Jacob RS, Omkumar RV. Mohanan AG, et al. Front Mol Neurosci. 2022 Jun 20;15:855752. doi: 10.3389/fnmol.2022.855752. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35795689 Free PMC article. Review.
References
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. - PubMed
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. - PubMed
- Cabelli RJ, Horn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous