Protein sorting in the Golgi complex - PubMed (original) (raw)
Review
Protein sorting in the Golgi complex
J Füllekrug et al. Biochim Biophys Acta. 1998.
Abstract
Even after one hundred years, the Golgi apparatus remains a major challenge in the field of Cell Biology. This is particularly true in terms of transport and of protein sorting. For example, the question how cargo proteins are transported through this organelle is still a matter of debate. Emphasis has been put on the role of anterograde and retrograde transport vesicles. These have been proposed to carry cargo from cisterna to cisterna and to recycle components needed for further rounds of transport. Alternatively, anterograde movement of cargo takes place in cisternal membranes rather than transport vesicles. These membranes assemble and mature in a cis to trans direction. In this case, retrograde transport vesicles need to recycle all components of the Golgi apparatus and this demands a highly dynamic and efficient sorting machinery. Here we will discuss possible mechanisms for protein sorting in the context of cisternal maturation and propose that a common mechanism is sufficient to explain both transport of cargo and sorting of resident proteins.
Figures
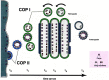
Fig. 1
How cisternal maturation would work. Importantly, this drawing depicts not spatial but time resolution. A given population of cargo molecules moves over time t0–t5 through the secretory pathway. t0: Cargo is selected and concentrated at ER exit sites with the help of COP II components. t1: COP II vesicles shed their coat and fuse with retrograde COP I vesicles carrying cis Golgi proteins, forming the first cisterna of the Golgi apparatus. t2: Cargo is modified by cis Golgi proteins, and these enzymes are recycling to fuse with COP II vesicles; at the same time, retrograde vesicles containing medial Golgi enzymes start to join the cargo containing cisterna. t3: Cargo is modified by medial Golgi proteins, and these enzymes are recycling to fuse with cis Golgi cisternae; at the same time, retrograde vesicles containing trans Golgi enzymes start to join the cargo containing cisterna. t4: Cargo is modified by trans Golgi enzymes, and these enzymes are recycling to fuse with medial cisternae; sorting and budding of vesicles result ultimately in the consumption of the trans cisterna. t5: Vesicles move to lysosomes, secretory granules or the plasma membrane. The grey bars at the cytosolic face of the individual cisternae correspond to the intracisternal matrix. If cargo molecules have a certain affinity for retrograde vesicles, they would undergo more than one round of maturation thus explaining different kinetics for transport from ER to plasma membrane. An essential feature of this maturation model is the prediction that Golgi resident proteins are more concentrated in retrograde vesicles than they are in the cisternae of the Golgi apparatus. Furthermore, since new cisternae are only assembled at the ER-Golgi intermediate compartment, every cisterna has to move forward to provide space for newly forming cisternae.
Similar articles
- Membrane cycling between the ER and Golgi apparatus and its role in biosynthetic transport.
Lippincott-Schwartz J. Lippincott-Schwartz J. Subcell Biochem. 1993;21:95-119. doi: 10.1007/978-1-4615-2912-5_5. Subcell Biochem. 1993. PMID: 8256276 Review. - S-Palmitoylation Sorts Membrane Cargo for Anterograde Transport in the Golgi.
Ernst AM, Syed SA, Zaki O, Bottanelli F, Zheng H, Hacke M, Xi Z, Rivera-Molina F, Graham M, Rebane AA, Björkholm P, Baddeley D, Toomre D, Pincet F, Rothman JE. Ernst AM, et al. Dev Cell. 2018 Nov 19;47(4):479-493.e7. doi: 10.1016/j.devcel.2018.10.024. Dev Cell. 2018. PMID: 30458139 Free PMC article. - The Golgi apparatus: going round in circles?
Barr FA. Barr FA. Trends Cell Biol. 2002 Mar;12(3):101-4. doi: 10.1016/s0962-8924(01)02240-1. Trends Cell Biol. 2002. PMID: 11859015 - Isolation of functional Golgi-derived vesicles with a possible role in retrograde transport.
Love HD, Lin CC, Short CS, Ostermann J. Love HD, et al. J Cell Biol. 1998 Feb 9;140(3):541-51. doi: 10.1083/jcb.140.3.541. J Cell Biol. 1998. PMID: 9456315 Free PMC article. - Protein sorting by directed maturation of Golgi compartments.
Allan BB, Balch WE. Allan BB, et al. Science. 1999 Jul 2;285(5424):63-6. doi: 10.1126/science.285.5424.63. Science. 1999. PMID: 10390362 Review.
Cited by
- Understanding the stepwise synthesis of glycolipids.
Maccioni HJ, Giraudo CG, Daniotti JL. Maccioni HJ, et al. Neurochem Res. 2002 Aug;27(7-8):629-36. doi: 10.1023/a:1020271932760. Neurochem Res. 2002. PMID: 12374198 Review. - Endoplasmic reticulum export of glycosyltransferases depends on interaction of a cytoplasmic dibasic motif with Sar1.
Giraudo CG, Maccioni HJ. Giraudo CG, et al. Mol Biol Cell. 2003 Sep;14(9):3753-66. doi: 10.1091/mbc.e03-02-0101. Epub 2003 May 18. Mol Biol Cell. 2003. PMID: 12972562 Free PMC article. - Quantitative analysis of intra-Golgi transport shows intercisternal exchange for all cargo.
Dmitrieff S, Rao M, Sens P. Dmitrieff S, et al. Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15692-7. doi: 10.1073/pnas.1303358110. Epub 2013 Sep 9. Proc Natl Acad Sci U S A. 2013. PMID: 24019488 Free PMC article. - Comprehensive Proteomic Characterization of the Intra-Golgi Trafficking Intermediates.
Sumya FT, Aragon-Ramirez WS, Lupashin VV. Sumya FT, et al. bioRxiv [Preprint]. 2024 Nov 12:2024.10.25.620336. doi: 10.1101/2024.10.25.620336. bioRxiv. 2024. PMID: 39484492 Free PMC article. Updated. Preprint. - Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport.
Martinez-Menárguez JA, Prekeris R, Oorschot VM, Scheller R, Slot JW, Geuze HJ, Klumperman J. Martinez-Menárguez JA, et al. J Cell Biol. 2001 Dec 24;155(7):1213-24. doi: 10.1083/jcb.200108029. Epub 2001 Dec 17. J Cell Biol. 2001. PMID: 11748250 Free PMC article.
References
- Grasse P.P. Acad. Sci. (Paris) 1957;245:1278–1281. - PubMed
- D.J. Morre, in B.R. Brinkley, K.R. Porter (Eds.), International Cell Biology, 1976–1977. Rockefeller University Press, New York, 1977, pp. 293–303.
- Rothman J.E., Wieland F.T. Protein sorting by transport vesicles. Science. 1996;272:227–234. - PubMed
- Schekman R., Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. - PubMed
- Melkonian M., Becker B., Becker D. Scale formation in algae. J. Electron Microsc. Tech. 1991;17:165–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources