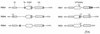Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes - PubMed (original) (raw)
. 1998 Sep;180(17):4416-25.
doi: 10.1128/JB.180.17.4416-4425.1998.
H C Wong, R Calhoon, D Gelfand, A L Fear, G Volman, R Mayer, P Ross, D Amikam, H Weinhouse, A Cohen, S Sapir, P Ohana, M Benziman
Affiliations
- PMID: 9721278
- PMCID: PMC107450
- DOI: 10.1128/JB.180.17.4416-4425.1998
Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes
R Tal et al. J Bacteriol. 1998 Sep.
Abstract
Cyclic di-GMP (c-di-GMP) is the specific nucleotide regulator of beta-1,4-glucan (cellulose) synthase in Acetobacter xylinum. The enzymes controlling turnover of c-di-GMP are diguanylate cyclase (DGC), which catalyzes its formation, and phosphodiesterase A (PDEA), which catalyzes its degradation. Following biochemical purification of DGC and PDEA, genes encoding isoforms of these enzymes have been isolated and found to be located on three distinct yet highly homologous operons for cyclic diguanylate, cdg1, cdg2, and cdg3. Within each cdg operon, a pdeA gene lies upstream of a dgc gene. cdg1 contains two additional flanking genes, cdg1a and cdg1d. cdg1a encodes a putative transcriptional activator, similar to AadR of Rhodopseudomonas palustris and FixK proteins of rhizobia. The deduced DGC and PDEA proteins have an identical motif structure of two lengthy domains in their C-terminal regions. These domains are also present in numerous bacterial proteins of undefined function. The N termini of the DGC and PDEA deduced proteins contain putative oxygen-sensing domains, based on similarity to domains on bacterial NifL and FixL proteins, respectively. Genetic disruption analyses demonstrated a physiological hierarchy among the cdg operons, such that cdg1 contributes 80% of cellular DGC and PDEA activities and cdg2 and cdg3 contribute 15 and 5%, respectively. Disruption of dgc genes markedly reduced in vivo cellulose production, demonstrating that c-di-GMP controls this process.
Figures
FIG. 1
Genetic organization of the cdg1, cdg2, and cdg3 operons and their flanking regions in A. xylinum, located on cosmid clones 3F3, 5C10, and 15A8, respectively. The pdeA and dgc genes are indicated by solid and open boxes, respectively. Arrows indicate the transcriptional directions of the genes.
FIG. 2
Primer extension analysis of operon cdg2. Total RNA extracted from A. xylinum 1306-3 was hybridized with 32P-end-labeled primer Cel-213. cDNA was synthesized with avian myeloblastosis virus reverse transcriptase and the four deoxyribonucleotide triphosphates. The length of the extension product (1) was measured against the terminal products produced in DNA sequencing reactions by using the same primer. G, A, T, and C indicate the individual sequencing reactions.
FIG. 3
Multiple alignment of the deduced amino acid sequences of the pdeA and dgc genes of A. xylinum showing the conserved GGDEF (regions I to IV) and EAL (regions V to VIII) domains. Residues identical in all six sequences are shaded in black and indicated in the consensus line; residues identical in five of six sequences are shaded in gray. In the region shown, the DGC and PDEA isoenzyme sets have 23 to 29% identity, compared to 77 to 89% identity among the PDEA sequences and 47% identity among the DGC sequences. Putative Q-linker regions are delimited by asterisks.
FIG. 4
Alignment of bacterial predicted protein sequences showing conservation of GGDEF and EAL domains. Residues with identity or conservative substitution in 100 and ≥80% of the sequences are shaded in black and gray, respectively. Identities displayed between each sequence and the A. xylinum (Ax) PDEA1 and DGC1 sequences, respectively, are shown in parentheses below. The amino acid sequences shown are from E. coli (Ec) YCIR, GenBank accession no. D90766 (50 and 28%); Bacillus subtilis (Bs) ykoW, GenBank Z99110 (50 and 26%); Synechocystis strain PCC6803 (Sy) slr0359, GenBank D63999 (49 and 30%); Rhizobium sp. strain NGR234 (Rh) Y4LL, GenBank AE000083 (48 and 32%); Mycobacterium tuberculosis (Mt) Y07I, GenBank Z75555 (31 and 32%).
FIG. 5
Multiple alignment of the N-terminal regions of the DGC1 and PDEA1 sequences showing conservation with domains on other proteins. Residues with identity or conservative substitution in 100 and ≥75% of the sequences are shaded in black and gray, respectively. (a) The amino acid sequences shown are from A. xylinum (Ax) DGC1, Synechocystis strain PCC6803 (Sy) slr1759 (sensory transduction histidine kinase, GenBank accession no. D90903), Klebsiella pneumoniae (Kp) NifL (GenBank X13303), E. coli (Ec) Aer (GenBank U28379), and Arabidopsis thaliana (At) NPH1 (GenBank AF030864). (b) Amino acid sequences shown are A. xylinum (Ax) PDEA1, E. coli (Ec) FixL (GenBank D90790), Rhizobium meliloti (Rm) FixL (GenBank Z70305), and Bradyrhizobium japonicum (Bj) FixL (GenBank P23222). An asterisk indicates a His residue conserved among FixL and PDEA proteins.
FIG. 6
Proposed domain structure of the PDEA and DGC isoenzymes of A. xylinum. The GGDEF and EAL domains form a motif conserved among all six isoenzymes. N-terminal regions putatively function in oxygen sensing (OS). Predicted sites of GTP binding may indicate catalytic sites of DGC proteins. TM, putative transmembrane helices; aa, amino acids.
Similar articles
- A flavin cofactor-binding PAS domain regulates c-di-GMP synthesis in AxDGC2 from Acetobacter xylinum.
Qi Y, Rao F, Luo Z, Liang ZX. Qi Y, et al. Biochemistry. 2009 Nov 3;48(43):10275-85. doi: 10.1021/bi901121w. Biochemistry. 2009. PMID: 19785462 - Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi.
Liu N, Xu Y, Hossain S, Huang N, Coursolle D, Gralnick JA, Boon EM. Liu N, et al. Biochemistry. 2012 Mar 13;51(10):2087-99. doi: 10.1021/bi201753f. Epub 2012 Mar 5. Biochemistry. 2012. PMID: 22360279 - Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3'-5')-cyclic-GMP in virulence.
Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. Kulasakara H, et al. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2839-44. doi: 10.1073/pnas.0511090103. Epub 2006 Feb 13. Proc Natl Acad Sci U S A. 2006. PMID: 16477007 Free PMC article. - [Activity of cyclic diguanylate (c-di-GMP) in bacteria and the study of its derivatives].
Na LX, Yang ZJ. Na LX, et al. Yao Xue Xue Bao. 2012 Mar;47(3):307-12. Yao Xue Xue Bao. 2012. PMID: 22645753 Review. Chinese. - Mechanisms of cyclic-di-GMP signaling in bacteria.
Jenal U, Malone J. Jenal U, et al. Annu Rev Genet. 2006;40:385-407. doi: 10.1146/annurev.genet.40.110405.090423. Annu Rev Genet. 2006. PMID: 16895465 Review.
Cited by
- A Pterin-Dependent Signaling Pathway Regulates a Dual-Function Diguanylate Cyclase-Phosphodiesterase Controlling Surface Attachment in Agrobacterium tumefaciens.
Feirer N, Xu J, Allen KD, Koestler BJ, Bruger EL, Waters CM, White RH, Fuqua C. Feirer N, et al. mBio. 2015 Jun 30;6(4):e00156. doi: 10.1128/mBio.00156-15. mBio. 2015. PMID: 26126849 Free PMC article. - Characterization of pellicle inhibition in Gluconacetobacter xylinus 53582 by a small molecule, pellicin, identified by a chemical genetics screen.
Strap JL, Latos A, Shim I, Bonetta DT. Strap JL, et al. PLoS One. 2011;6(12):e28015. doi: 10.1371/journal.pone.0028015. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174763 Free PMC article. - Phenotypic convergence mediated by GGDEF-domain-containing proteins.
Simm R, Fetherston JD, Kader A, Römling U, Perry RD. Simm R, et al. J Bacteriol. 2005 Oct;187(19):6816-23. doi: 10.1128/JB.187.19.6816-6823.2005. J Bacteriol. 2005. PMID: 16166544 Free PMC article. - Analysis of cellulose synthesis in a high-producing acetic acid bacterium Komagataeibacter hansenii.
Bimmer M, Reimer M, Klingl A, Ludwig C, Zollfrank C, Liebl W, Ehrenreich A. Bimmer M, et al. Appl Microbiol Biotechnol. 2023 May;107(9):2947-2967. doi: 10.1007/s00253-023-12461-z. Epub 2023 Mar 17. Appl Microbiol Biotechnol. 2023. PMID: 36930278 Free PMC article. - The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains.
Schmidt AJ, Ryjenkov DA, Gomelsky M. Schmidt AJ, et al. J Bacteriol. 2005 Jul;187(14):4774-81. doi: 10.1128/JB.187.14.4774-4781.2005. J Bacteriol. 2005. PMID: 15995192 Free PMC article.
References
- Akiyama M, Crooke E, Kornberg A. An exopolyphosphatase of Escherichia coli. J Biol Chem. 1993;268:633–639. - PubMed
- Aloni Y, Cohen Y, Benziman M, Delmer D P. Solubilization of UDP-glucose: 1,4-β-d-glucan 4-β-d-glucosyl transferase (cellulose synthase) from Acetobacter xylinum. J Biol Chem. 1983;258:4419–4423. - PubMed
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources