Microinjection of anti-coilin antibodies affects the structure of coiled bodies - PubMed (original) (raw)
Microinjection of anti-coilin antibodies affects the structure of coiled bodies
F Almeida et al. J Cell Biol. 1998.
Abstract
The coiled body is a distinct subnuclear domain enriched in small nuclear ribonucleoprotein particles (snRNPs) involved in processing of pre-mRNA. Although the function of the coiled body is still unknown, current models propose that it may have a role in snRNP biogenesis, transport, or recycling. Here we describe that anti-coilin antibodies promote a specific disappearance of the coiled body in living human cells, thus providing a novel tool for the functional analysis of this structure. Monoclonal antibodies (mAbs) were raised against recombinant human coilin, the major structural protein of the coiled body. Four mAbs are shown to induce a progressive disappearance of coiled bodies within approximately 6 h after microinjection into the nucleus of HeLa cells. After their disappearance, coiled bodies are not seen to re-form, although injected cells remain viable for at least 3 d. Epitope mapping reveals that the mAbs recognize distinct amino acid motifs scattered along the complete coilin sequence. By 24 and 48 h after injection of antibodies that promote coiled body disappearance, splicing snRNPs are normally distributed in the nucleoplasm, the nucleolus remains unaffected, and the cell cycle progresses normally. Furthermore, cells devoid of coiled bodies for approximately 24 h maintain the ability to splice both adenoviral pre-mRNAs and transiently overexpressed human beta-globin transcripts. In conclusion, within the time range of this study, no major nuclear abnormalities are detected after coiled body disappearance.
Figures
Figure 1
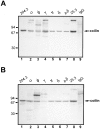
Immunoblot analysis of anti-coilin mAbs. An 8% acrylamide gel was loaded with either total protein extract from HeLa cells (2 × 105 cells/well) (A) or a protein extract from isolated HeLa cell nuclei (106 cell equivalents/well) (B). Immunoblot analysis was performed using the following antibodies: rabbit antiserum 204.3 (diluted 1:20,000) (lane 1); mAb-o (25 μg/ml purified IgG) (lane 2); mAb-φ (10 μg/ml purified IgG) (lane 3); mAb-γ (25 μg/ml purified IgG) (lane 4); mAb-π (5 μg/ml purified IgG) (lane 5); mAb-δ (0.08 μg/ml purified IgG) (lane 6); mAb-pδ (15 μg/ml purified IgG) (lane 7); mouse antiserum 25.3 (diluted 1:5,000) (lane 8); and nonspecific polyclonal mouse IgG (5 μg/ml purified IgG) (lane 9). Due to significant differences in the binding affinity of each mAb, different antibody concentrations were used in order to produce a signal of similar intensity. Antiserum 25.3 derives from the mouse used to produce hybridomas φ, γ, π, δ, and pδ. Nonspecific mouse IgG was purchased from Sigma Chemical Co. Molecular mass markers (kD) are indicated on the left.
Figure 2
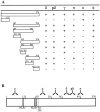
Epitope mapping of mAbs. (A) Diagram showing the reactivity of each mAb with the full-length coilin and different deletion mutants of the protein, as determined by immunoblot analysis. The data show that the epitopes recognized by mAbs o, φ, and γ map between amino acids 1–127, 187–291, and 292–362, respectively. The mAbs δ and pδ react with epitopes located between acids 363 and 481. The mAb-π recognizes either an epitope localized around amino acids 481–482, or a conformational epitope present in the coilin mutant encompassing amino acids 363–576, but absent from the mutants encompassing amino acids 363–481 and 482–576. (B) Diagram showing the mapped epitopes (𝕐) in relation to the complete coilin sequence. Hatched regions depict the two motifs that closely match the consensus sequence of simple and bipartite nuclear localization sequences, NLSa and NLSb, respectively (Bohmann et al., 1995_a_). The diagram also depicts the position of serine 202. Conversion of this amino acid residue into aspartate causes the disappearance of coiled bodies and a redistribution of coilin to intranucleolar domains (Lyon et al., 1997).
Figure 3
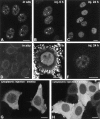
Microinjection mAb-δ. (A) HeLa cells were permeabilized with Triton X-100, fixed with formaldehyde, and then incubated with mAb-δ. (B and C) HeLa cells were microinjected in the nucleus with mAb-δ. The cells were then observed immediately (B) or 24 h after injection (C). (D–F) Purified mAb-δ was incubated for 1 h with 1:2 molar excess of a coilin peptide containing the epitope for this antibody (amino acids 363–481). The neutralized mAb was then used for indirect immunofluorescence on fixed cells (D) or microinjected in the nucleus of living cells (E and F). (E) Immediately after injection the neutralized mAb is diffusely distributed throughout the nucleoplasm with additional staining of a coiled body (arrow). Note the bright pericellular staining which is due to antibody–antigen complexes adsorbed to the cell membrane. (F) 24 h after injection the staining is predominantly concentrated in coiled bodies. (G and H) HeLa cells were microinjected in the cytoplasm with mAb-δ and observed 1 h after injection. The cells were either nontreated (G) or treated with 10 μg/ml emetine for 2.5 h before injection (H). Bar, 10 μm.
Figure 4
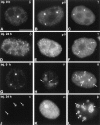
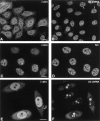
The effect of mAb injection on coiled bodies. HeLa cells were microinjected in the nucleus with mAbs δ (A and D), pδ (B and E), γ (C and F), π (G and J), o (H and K), and φ (I and L). The cells were observed either immediately after injection (A–C, G–I) or 24 h after injection (D–F, J–L). For microscopical observation, the cells were permeabilized with Triton X-100, fixed, and incubated with a secondary antibody coupled to fluorescein. Note that mAbs o and φ label both coiled bodies (arrows) and nucleoli (arrowheads). Bar, 10 μm.
Figure 5
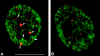
Coilin does not colocalize with transcription sites. (A) HeLa cells were microinjected with Br-UTP in the cytoplasm, fixed, and then immunolabeled with mAb-π. The sites of incorporated Br-uridine are stained green and the sites containing coilin are stained red. Note that coilin is present both in coiled bodies (arrows) and in numerous nucleoplasmic microfoci (arrowheads). (B) HeLa cells were injected in the nucleus with mAb-δ. 24 h later the cells were reinjected in the cytoplasm with Br-UTP. Note that the coilin microfoci (stained red) do not colocalize with transcription sites (stained green). Bar, 10 μm.
Figure 6
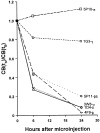
Quantitative analysis of the effects of mAb injection on coiled bodies. Cells were microinjected in the nucleus with mAbs δ, pδ, γ, π, o, and φ. The cells were either immediately permeabilized and fixed (t0), or further incubated for 6 or 24 h. The injected mAb was detected using fluorescein-conjugated secondary antibodies. Cells were double-labeled with a rabbit anti-coilin antiserum (204.3) detected using Texas red fluorescence. For each mAb, the percentage of injected cells with coiled bodies was estimated at each time point (CB[tn]), and the values at time zero (CB[t0]) were taken as reference. For each mAb, at least three independent microinjection experiments were performed. The total number of cells counted per mAb at each time point ranged between 100 and 600.
Figure 7
Analysis of mAb-π in living cells. HeLa cells were injected with rhodamine-labeled mAb-π and analyzed at 30-min intervals. All cells in this field were injected in the nucleus, except one that was injected in the cytoplasm (A and B, arrowheads). The time between injection and the first observation was ∼20 min. Note that shortly after injection, the antibody injected in the cytoplasm (A and B, arrowheads) labels coiled bodies (A–D, arrows). At 1.5 h after the first observation, one cell initially injected in the nucleus has entered mitosis (arrowhead), and 3 h later coiled bodies are labeled in the daughter cell nuclei (E, arrows). At 6 h after the first observation, another cell is in mitosis and contains a labeled mitotic coiled body in the cytoplasm (F, arrowhead). Bar, 10 μm.
Figure 8
Analysis of mAb-δ in living cells. HeLa cells were injected with rhodamine-labeled mAb-δ and analyzed at 30-min intervals. All cells in this field were injected in the nucleus. The time between injection and the first observation was ∼20 min. Within 6 h after the first observation, the coiled bodies progressively disappear (A–G). At later time points coiled bodies are never detected in these cells (H–L). Note that one of the cells has divided by 39 h (L, arrows). J and L depict phase-contrast images corresponding to I and K, respectively. Bar, 10 μm.
Figure 9
Coiled body disappearance does not affect snRNP localization. HeLa cells were microinjected with mAb-δ and incubated for either 24 (A–D) or 48 h (E and F) before fixation. The injected antibody was detected using a secondary antibody conjugated to Texas red (A, C, and E). The cells were double-labeled with either an antisense riboprobe specific for U2 snRNA (B), an antibody against the Sm proteins (D), or an antisense riboprobe specific for U3 snoRNA (F). Arrows in B point to U2 snRNA concentrated in the coiled bodies of noninjected cells. Bar, 10 μm.
Figure 10
Coiled body disappearance does not impair splicing. (A–D) HeLa cells were microinjected with mAb-δ, incubated for either 6 (A and B) or 24 h (C and D) and then infected with Ad2 for 18 h. The cells were fixed and hybridized with an oligonucleotide probe complementary to the first splice junction of the tripartite leader (A and C). The injected antibody was detected using a secondary antibody conjugated to Texas red (B and D), and the viral protein DBP was detected using a secondary antibody conjugated to Cy5 (data not shown). The hybridization pattern in the injected cells (A, arrow; and C) is similar to that of noninjected cells (A, arrowheads). Note that in B and D, staining of the nucleoplasm is nonhomogeneous. This is due to the very intense signal produced by anti-DBP antibody, which is partially detected in the Texas red channel of the confocal microscope. (E and F) Cells were microinjected with mAb 1D4-δ, incubated for 24 h, and then re-injected with a expression plasmid carrying the human β-globin gene. The cells were fixed 16 h later and hybridized with an oligonucleotide probe complementary to the first splice junction of β-globin mRNA (E). The injected antibody was detected using a secondary IgG conjugated to Texas red (F). Note that under the mild permeabilization conditions used here, the hybridization signal is predominantly detected in the cytoplasm. Bar, 10 μm.
Similar articles
- Dynamic interactions between splicing snRNPs, coiled bodies and nucleoli revealed using snRNP protein fusions to the green fluorescent protein.
Sleeman J, Lyon CE, Platani M, Kreivi JP, Lamond AI. Sleeman J, et al. Exp Cell Res. 1998 Sep 15;243(2):290-304. doi: 10.1006/excr.1998.4135. Exp Cell Res. 1998. PMID: 9743589 - Coiled bodies without coilin.
Bauer DW, Gall JG. Bauer DW, et al. Mol Biol Cell. 1997 Jan;8(1):73-82. doi: 10.1091/mbc.8.1.73. Mol Biol Cell. 1997. PMID: 9017596 Free PMC article. - Inhibition of protein dephosphorylation results in the accumulation of splicing snRNPs and coiled bodies within the nucleolus.
Lyon CE, Bohmann K, Sleeman J, Lamond AI. Lyon CE, et al. Exp Cell Res. 1997 Jan 10;230(1):84-93. doi: 10.1006/excr.1996.3380. Exp Cell Res. 1997. PMID: 9013710 - The Cajal body.
Morris GE. Morris GE. Biochim Biophys Acta. 2008 Nov;1783(11):2108-15. doi: 10.1016/j.bbamcr.2008.07.016. Epub 2008 Aug 3. Biochim Biophys Acta. 2008. PMID: 18755223 Review. - Cajal bodies in neurons.
Lafarga M, Tapia O, Romero AM, Berciano MT. Lafarga M, et al. RNA Biol. 2017 Jun 3;14(6):712-725. doi: 10.1080/15476286.2016.1231360. Epub 2016 Sep 14. RNA Biol. 2017. PMID: 27627892 Free PMC article. Review.
Cited by
- The spinal muscular atrophy disease gene product, SMN: A link between snRNP biogenesis and the Cajal (coiled) body.
Carvalho T, Almeida F, Calapez A, Lafarga M, Berciano MT, Carmo-Fonseca M. Carvalho T, et al. J Cell Biol. 1999 Nov 15;147(4):715-28. doi: 10.1083/jcb.147.4.715. J Cell Biol. 1999. PMID: 10562276 Free PMC article. - TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture.
Herold A, Suyama M, Rodrigues JP, Braun IC, Kutay U, Carmo-Fonseca M, Bork P, Izaurralde E. Herold A, et al. Mol Cell Biol. 2000 Dec;20(23):8996-9008. doi: 10.1128/MCB.20.23.8996-9008.2000. Mol Cell Biol. 2000. PMID: 11073998 Free PMC article. - Dynamic association and localization of human H/ACA RNP proteins.
Kittur N, Darzacq X, Roy S, Singer RH, Meier UT. Kittur N, et al. RNA. 2006 Dec;12(12):2057-62. doi: 10.1261/rna.249306. Epub 2006 Oct 24. RNA. 2006. PMID: 17135485 Free PMC article. - Distribution of different phosphorylated forms of RNA polymerase II in relation to Cajal and PML bodies in human cells: an ultrastructural study.
Xie SQ, Pombo A. Xie SQ, et al. Histochem Cell Biol. 2006 Jan;125(1-2):21-31. doi: 10.1007/s00418-005-0064-2. Epub 2005 Sep 27. Histochem Cell Biol. 2006. PMID: 16187066 - A novel EB-1/AIDA-1 isoform, AIDA-1c, interacts with the Cajal body protein coilin.
Xu H, Hebert MD. Xu H, et al. BMC Cell Biol. 2005 Apr 29;6(1):23. doi: 10.1186/1471-2121-6-23. BMC Cell Biol. 2005. PMID: 15862129 Free PMC article.
References
- Ansorge W, Pepperkok R. Performance of an automated system for capillary microinjection into living cells. J Biochem Biophys Methods. 1988;16:283–292. - PubMed
- Beven AF, Simpson GG, Brown JWS, Shaw PJ. The organization of spliceosomal components in the nuclei of higher plants. J Cell Sci. 1995;108:509–518. - PubMed