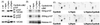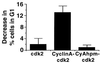Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A - PubMed (original) (raw)
Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A
B A Schulman et al. Proc Natl Acad Sci U S A. 1998.
Abstract
An important question in the cell cycle field is how cyclin-dependent kinases (cdks) target their substrates. We have studied the role of a conserved hydrophobic patch on the surface of cyclin A in substrate recognition by cyclin A-cdk2. This hydrophobic patch is approximately 35A away from the active site of cdk2 and contains the MRAIL sequence conserved among a number of mammalian cyclins. In the x-ray structure of cyclin A-cdk2-p27, this hydrophobic patch contacts the RNLFG sequence in p27 that is common to a number of substrates and inhibitors of mammalian cdks. We find that mutation of this hydrophobic patch on cyclin A eliminates binding to proteins containing RXL motifs without affecting binding to cdk2. This docking site is critical for cyclin A-cdk2 phosphorylation of substrates containing RXL motifs, but not for phosphorylation of histone H1. Impaired substrate binding by the cyclin is the cause of the defect in RXL substrate phosphorylation, because phosphorylation can be rescued by restoring a cyclin A-substrate interaction in a heterologous manner. In addition, the conserved hydrophobic patch is important for cyclin A function in cells, contributing to cyclin A's ability to drive cells out of the G1 phase of the cell cycle. Thus, we define a mechanism by which cyclins can recruit substrates to cdks, and our results support the notion that a high local concentration of substrate provided by a protein-protein interaction distant from the active site is critical for phosphorylation by cdks.
Figures
Figure 1
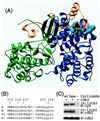
Structural basis for interactions with cyclin A. (A) Crystal structure of cyclin A (blue) -cdk2 (green) -p27 (yellow) (35). The residues mutated in this report are in light-blue space-filling representation. The RXL motif (R30, L32, and F33) involved important in p27–cyclin interactions is shown in transparent red space-filling representation. (B) The sequence in cyclin A that contacts p27 is conserved among a number of mammalian cyclins. Residues mutated in this report are marked with ∗. (C) The hydrophobic patch mutant binds cdk2 when immunoprecipitated from lysates of U2OS cells cotransfected with plasmids expressing HA-tagged cyclin A and cdk2. A representative experiment is shown. In a series of similar experiments, the amount of cdk2 present in 12CA5 immunoprecipitates is proportional to the amount of either HA-tagged cyclin A or cyAhpm.
Figure 2
The hydrophobic patch on cyclin A recruits RXL proteins. (A) Binding to p107 was assayed by immunoprecipitation and Western blotting of p107 or HA-tagged cyclin A in lysates from transfected U2OS cells. (B) Binding to E2F1 was assayed by immunoprecipitation and Western blotting of E2F1 or HA-tagged cyclin A. Lysates from U2OS cells transfected with either cyclin A- or cyAhpm-cdk2 were mixed with recombinant E2F1 before immunoprecipitation. (C) Binding to p21 was detected by Western blotting for Myc-tagged cyclin A or cdk2 after immunoprecipitation of p21 from transfected U2OS cells. (D) Binding to p27 was assayed by glutathione-agarose precipitation of GST or GST-p27 and Western blotting for Myc-tagged cyclin A or cdk2. Lysates from U2OS cells transfected with either cyclin A or cyAhpm and/or cdk2 were mixed with recombinant GST or GST-p27 before precipitation.
Figure 3
The hydrophobic patch on cyclin A is important for phosphorylation of a subset of substrates. HA-tagged cyclin A–cdk2 or cyAhpm–cdk2 complexes immunoprecipitated from transfected U2OS cells were incubated with [γ-32P]ATP and the following amounts of the following substrates: histone H1, 100 ng, 250 ng, 1 μg, and 2 μg; p107, 10 ng, 50 ng, 200 ng, and 500 ng; E2F1, 10 ng, 50 ng, 200 ng, and 500 ng; Rb-C, 10 ng, 50 ng, 200 ng, and 1 μg. Autophosphorylated cyclin A is indicated with ∗.
Figure 4
Cyclin A binding is important for cdk2 phosphorylation of an RXL substrate. (A) Binding to p107 by LXCXE-hpm mutants of cyclin A was tested by immunoprecipitation of p107 and Western blotting of HA-tagged cyclin A from lysates of transfected U2OS cells. (B) L1hpm– and L2hpm–cdk2 complexes were tested as in Fig. 3 for their ability to phosphorylate the indicated amounts of p107 or 500 ng of histone H1. (C) Phosphopeptide maps of p107 phosphorylated by wild-type cyclin A-, L1hpm- and L2hpm-cdk2 in B.
Figure 5
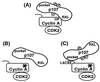
A model for cyclin A-cdk2 recognition of a substrate, p107. (A) The RXL motif in p107 normally contacts the hydrophobic patch in cyclin A. (B) Mutation of the hydrophobic patch on cyclin A dramatically reduces binding to and phosphorylation of p107. (C) Binding between p107 and a hydrophobic patch mutant of cyclin A can be restored by addition of the p107 pocket-binding sequence, LXCXE, to cyclin A. Restoration of binding is sufficient to rescue phosphorylation. Thus, a specific trajectory between substrate binding and the kinase active site is not necessary for phosphorylation, and the purpose of substrate binding is to increase the local concentration available to the kinase.
Figure 6
Decrease in G1 population of U2OS cells transiently expressing either cdk2 alone, cyclin A-cdk2, or cyAhpm-cdk2. The results are the mean of at least three independent experiments, and the error bars indicate SD from the mean. The baseline G1 fraction in vector-transfected cells is 32.8%.
Similar articles
- Specificity determinants of recruitment peptides bound to phospho-CDK2/cyclin A.
Lowe ED, Tews I, Cheng KY, Brown NR, Gul S, Noble ME, Gamblin SJ, Johnson LN. Lowe ED, et al. Biochemistry. 2002 Dec 31;41(52):15625-34. doi: 10.1021/bi0268910. Biochemistry. 2002. PMID: 12501191 - The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases.
Brown NR, Noble ME, Endicott JA, Johnson LN. Brown NR, et al. Nat Cell Biol. 1999 Nov;1(7):438-43. doi: 10.1038/15674. Nat Cell Biol. 1999. PMID: 10559988 - Cyclin B and cyclin A confer different substrate recognition properties on CDK2.
Brown NR, Lowe ED, Petri E, Skamnaki V, Antrobus R, Johnson LN. Brown NR, et al. Cell Cycle. 2007 Jun 1;6(11):1350-9. doi: 10.4161/cc.6.11.4278. Epub 2007 Jun 11. Cell Cycle. 2007. PMID: 17495531 - [The characterization of human cdc2 kinase and CDK2].
Yasuda H, Kamijo M, Ohba Y. Yasuda H, et al. Yakugaku Zasshi. 1993 Dec;113(12):829-46. doi: 10.1248/yakushi1947.113.12_829. Yakugaku Zasshi. 1993. PMID: 8301538 Review. Japanese. - Structuring cell-cycle biology.
Levine K, Cross FR. Levine K, et al. Structure. 1995 Nov 15;3(11):1131-4. doi: 10.1016/s0969-2126(01)00248-9. Structure. 1995. PMID: 8591023 Review.
Cited by
- CDK activity at the centrosome regulates the cell cycle.
Roberts EL, Greenwood J, Kapadia N, Auchynnikava T, Basu S, Nurse P. Roberts EL, et al. Cell Rep. 2024 Apr 23;43(4):114066. doi: 10.1016/j.celrep.2024.114066. Epub 2024 Apr 4. Cell Rep. 2024. PMID: 38578823 Free PMC article. - An Update on Protein Kinases as Therapeutic Targets-Part II: Peptides as Allosteric Protein Kinase C Modulators Targeting Protein-Protein Interactions.
Zerihun M, Rubin SJS, Silnitsky S, Qvit N. Zerihun M, et al. Int J Mol Sci. 2023 Dec 15;24(24):17504. doi: 10.3390/ijms242417504. Int J Mol Sci. 2023. PMID: 38139336 Free PMC article. Review. - CDK signaling via nonconventional CDK phosphorylation sites.
Valk E, Örd M, Faustova I, Loog M. Valk E, et al. Mol Biol Cell. 2023 Nov 1;34(12):pe5. doi: 10.1091/mbc.E22-06-0196. Mol Biol Cell. 2023. PMID: 37906435 Free PMC article. Review. - The E3 Ubiquitin Ligase SCF Cyclin F Promotes Sequestosome-1/p62 Insolubility and Foci Formation and is Dysregulated in ALS and FTD Pathogenesis.
Davidson JM, Wu SSL, Rayner SL, Cheng F, Duncan K, Russo C, Newbery M, Ding K, Scherer NM, Balez R, García-Redondo A, Rábano A, Rosa-Fernandes L, Ooi L, Williams KL, Morsch M, Blair IP, Di Ieva A, Yang S, Chung RS, Lee A. Davidson JM, et al. Mol Neurobiol. 2023 Sep;60(9):5034-5054. doi: 10.1007/s12035-023-03355-2. Epub 2023 May 27. Mol Neurobiol. 2023. PMID: 37243816 Free PMC article. - ALS-linked loss of Cyclin-F function affects HSP90.
Siebert A, Gattringer V, Weishaupt JH, Behrends C. Siebert A, et al. Life Sci Alliance. 2022 Sep 16;5(12):e202101359. doi: 10.26508/lsa.202101359. Life Sci Alliance. 2022. PMID: 36114006 Free PMC article.
References
- Hunt T. Curr Opin Cell Biol. 1989;1:268–274. - PubMed
- Nurse P. Nature (London) 1990;344:503–508. - PubMed
- Norbury C, Nurse P. Annu Rev Biochem. 1992;61:441–470. - PubMed
- Pines J, Hunter T. Ciba Found Symp. 1992;170:187–196. - PubMed
- Nasmyth K. Curr Opin Cell Biol. 1993;5:166–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources