The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase - PubMed (original) (raw)
The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase
R Ferreira et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The transcription factor E2F plays a major role in cell cycle control in mammalian cells. E2F binding sites, which are present in the promoters of a variety of genes required for S phase, shift from a negative to a positive role in transcription at the commitment point, a crucial point in G1 that precedes the G1/S transition. Before the commitment point, E2F activity is repressed by members of the pocket proteins family. This repression is believed to be crucial for the proper control of cell growth. We have previously shown that Rb, the founding member of the pocket proteins family, represses E2F1 activity by recruiting the histone deacetylase HDAC1. Here, we show that the two other members of the pocket proteins family, p107 and p130, also are able to interact physically with HDAC1 in live cells. HDAC1 interacts with p107 and Rb through an "LXCXE"-like motif, similar to that used by viral transforming proteins to bind and inactivate pocket proteins. Indeed, we find that the viral transforming protein E1A competes with HDAC1 for p107 interaction. We also demonstrate that p107 is able to interact simultaneously with HDAC1 and E2F4, suggesting a model in which p107 recruits HDAC1 to repress E2F sites. Indeed, we demonstrate that histone deacetylase activity is involved in the p107- or p130-induced repression of E2F4. Taken together, our data suggest that all members of the E2F family are regulated in early G1 by similar complexes, containing a pocket protein and the histone deacetylase HDAC1.
Figures
Figure 1
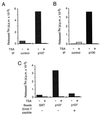
p107 and p130 interact with HDAC1 (A) p107 and p130 interact with HDAC1 in live cells. Total cell extracts of SAOS2 cells transfected with the indicated expression vectors were immunoprecipitated with the indicated antibody (Irr: anti-Flag antibody). The amount of CMV promoter in the transfection was kept constant by using empty vectors. Immunoprecipitates were assayed for the presence of transfected HA-HDAC1 by Western blot by using the anti-HA antibody. In lanes 1, 4, 6, and 8, only one-third of immunoprecipitates was loaded onto the gel. (B) p107 interacts with HDAC1 in vitro. 35S-labeled, _in vitro_-translated HDAC1 was subjected to a GST pull-down analysis with beads harboring the indicated GST fusion protein. Bound proteins were then separated by SDS/PAGE analysis and visualized by autoradiography. In lane 1, 10% of the input HDAC1 was loaded directly onto the gel. (C) The pocket domain of p107 is required for binding to HDAC1. Labeled, _in vitro_-translated HDAC1 was subjected to a GST pull-down analysis with beads harboring either wild-type p107 (lane 3), a point mutant of p107 (p107 m, p107 713 C-F) (lane 4), or GST as a control (lane 2).
Figure 2
p107 interacts with the LXCXE-like motif of HDAC1 (A) The IACEE sequence of HDAC1 is required for p107 binding. _In vitro_-translated, labeled HDAC1 (lanes 1–4) or HDAC1 ΔIACEE (lanes 5–8) was subjected to a GST pull-down analysis by using beads covered with the indicated GST fusion protein. (B) LXCXE motif-containing peptides inhibit p107/HDAC1 interaction. _In vitro_-translated labeled HDAC1 was subjected to a GST pull-down analysis by using beads covered with GST (lane 2) or GST-p107 fusion protein. Before GST pull-down analysis, beads were incubated without (lane 3) or with 15 μg of control (lane 4), SV40 T (lane 5), or HDAC1 (lane 6) peptide.
Figure 3
p107 and p130 interact with a cellular histone deacetylase. (A) p107 is associated physically with a histone deacetylase. Nuclear extracts from U937 cells were immunoprecipitated with an anti-p107 (p107) or anti-HA (control) antibodies. Immunoprecipitates then were assayed for the presence of histone deacetylase activity. (B) p130 is associated physically with a histone deacetylase. Whole cell extracts from Jurkat cells were immunoprecipitated with anti-p130 (p130) or anti-HA (control) antibodies. Immunoprecipitates then were assayed for the presence of histone deacetylase activity. (C) p107 interacts with a cellular histone deacetylase through an LXCXE-like motif. Nuclear extracts from Jurkat cells were subjected to a GST pull-down analysis with beads covered with the indicated GST fusion protein. Before the incubation with nuclear extracts, beads were incubated with 100 μg of SV40 T peptide where indicated or 100 μg of control peptide. Bound proteins were assayed for the presence of deacetylase activity.
Figure 4
p107 interacts with E2F4 and HDAC1 simultaneously. Total cell extracts of SAOS2 cells transfected with the indicated expression vectors were immunoprecipitated with the indicated antibody. The amount of CMV promoter was kept constant in the transfection by using empty vectors. Immunoprecipitates were assayed for the presence of transfected HA-HDAC1 by Western blot by using the anti-HA antibody. In lanes 1, 4, and 5, only one-third of the immunoprecipitates was loaded onto the gel.
Figure 5
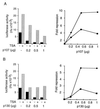
p107 and p130 repress E2F4 activity through a histone deacetylase. (A) SAOS2 cells were transfected transiently with 2 μg of E2F-luc reporter vector, 100 ng of pcDNA3 myc-E2F4, 100 ng of pCMV DP2, and the indicated dose of pCMV p107. The amount of CMV promoter was kept constant by using empty vectors. TSA (100 ng/ml) was added (gray bars) or not (black bars) 8 h later, and luciferase activity was assayed 24 h after transfection. Result of a typical experiment is shown on the Left. The mean of fold repression by p107 calculated from various independent experiments in the absence (circles) or in the presence (squares) of TSA is shown on the Right. (B) SAOS2 cells were transiently transfected and assayed as in A with the indicated dose of CMV p130.
Figure 6
Inactivation of p107 results in the loss of HDAC1 binding. (A) Cyclin D-dependent kinase expression inhibits the p107/HDAC1 interaction. Total cell extracts of SAOS2 cells transfected with the HA-HDAC1 and the p107, cyclin D1, cdk4, cyclin E, or cdk2 expression vectors as indicated were immunoprecipitated with the anti-p107 antibody (Upper) or the anti-HA antibody (Lower). Immunoprecipitates were assayed for the presence of HA-HDAC1 by Western blot by using the anti-HA antibody. (B) E1A competes with HDAC1 for p107 binding. _In vitro-_translated, labeled HDAC1 was subjected to a GST pull-down analysis by using beads covered with the indicated GST fusion protein. Before the GST pull down, p107 beads (lanes 3 and 4) and Rb beads (lanes 5 and 6) were incubated with or without purified recombinant GST-E1A 13S (50 ng) as indicated. (C) E1A competes with the cellular histone deacetylase for binding to p107 Jurkat nuclear extracts were subjected to a GST pull-down analysis by using beads covered with the indicated GST fusion protein. Before the GST pull-down, GST-p107 beads were incubated with or without purified GST-E1A 13S protein (300 ng) as indicated. Bound proteins were assayed for the presence of histone deacetylase activity. (D) Model of transcriptional repression through E2F sites. A heterodimer comprised of one E2F and one DP protein recognizes the E2F site. The E2F protein binds to a member of the pocket protein family, which recruits the HDAC1 deacetylase to the promoter to block transcription.
Similar articles
- The p107 tumor suppressor induces stable E2F DNA binding to repress target promoters.
O'Connor RJ, Schaley JE, Feeney G, Hearing P. O'Connor RJ, et al. Oncogene. 2001 Apr 5;20(15):1882-91. doi: 10.1038/sj.onc.1204278. Oncogene. 2001. PMID: 11313936 - E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor beta through E2F binding sites.
Li JM, Hu PP, Shen X, Yu Y, Wang XF. Li JM, et al. Proc Natl Acad Sci U S A. 1997 May 13;94(10):4948-53. doi: 10.1073/pnas.94.10.4948. Proc Natl Acad Sci U S A. 1997. PMID: 9144170 Free PMC article. - E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex.
Rayman JB, Takahashi Y, Indjeian VB, Dannenberg JH, Catchpole S, Watson RJ, te Riele H, Dynlacht BD. Rayman JB, et al. Genes Dev. 2002 Apr 15;16(8):933-47. doi: 10.1101/gad.969202. Genes Dev. 2002. PMID: 11959842 Free PMC article. - Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation.
Sidle A, Palaty C, Dirks P, Wiggan O, Kiess M, Gill RM, Wong AK, Hamel PA. Sidle A, et al. Crit Rev Biochem Mol Biol. 1996 Jun;31(3):237-71. doi: 10.3109/10409239609106585. Crit Rev Biochem Mol Biol. 1996. PMID: 8817077 Review. - Role of the retinoblastoma protein family, pRB, p107 and p130 in the negative control of cell growth.
Graña X, Garriga J, Mayol X. Graña X, et al. Oncogene. 1998 Dec 24;17(25):3365-83. doi: 10.1038/sj.onc.1202575. Oncogene. 1998. PMID: 9916999 Review.
Cited by
- Mechanistic Sequence of Histone Deacetylase Inhibitors and Radiation Treatment: An Overview.
Seane EN, Nair S, Vandevoorde C, Joubert A. Seane EN, et al. Pharmaceuticals (Basel). 2024 May 8;17(5):602. doi: 10.3390/ph17050602. Pharmaceuticals (Basel). 2024. PMID: 38794172 Free PMC article. Review. - HDAC activity is dispensable for repression of cell-cycle genes by DREAM and E2F:RB complexes.
Barrett AK, Shingare MR, Rechtsteiner A, Rodriguez KM, Le QN, Wijeratne TU, Mitchell CE, Membreno MW, Rubin SM, Müller GA. Barrett AK, et al. Nat Commun. 2024 May 24;15(1):4450. doi: 10.1038/s41467-024-48724-0. Nat Commun. 2024. PMID: 38789411 Free PMC article. - The p21CIP1-CDK4-DREAM axis is a master regulator of genotoxic stress-induced cellular senescence.
Schmidt A, Allmann S, Schwarzenbach C, Snyder P, Chen JX, Nagel G, Schöneis A, Rasenberger B, Beli P, Loewer A, Hofmann TG, Tomicic MT, Christmann M. Schmidt A, et al. Nucleic Acids Res. 2024 Jul 8;52(12):6945-6963. doi: 10.1093/nar/gkae426. Nucleic Acids Res. 2024. PMID: 38783095 Free PMC article. - HDAC activity is dispensable for repression of cell-cycle genes by DREAM and E2F:RB complexes.
Barrett A, Shingare MR, Rechtsteiner A, Wijeratne TU, Rodriguez KM, Rubin SM, Müller GA. Barrett A, et al. bioRxiv [Preprint]. 2023 Oct 28:2023.10.28.564489. doi: 10.1101/2023.10.28.564489. bioRxiv. 2023. PMID: 37961464 Free PMC article. Updated. Preprint. - Preliminary Characterization of the Epigenetic Modulation in the Human Mesenchymal Stem Cells during Chondrogenic Process.
Miceli M, Maruotti GM, Sarno L, Carbone L, Guida M, Pelagalli A. Miceli M, et al. Int J Mol Sci. 2022 Aug 30;23(17):9870. doi: 10.3390/ijms23179870. Int J Mol Sci. 2022. PMID: 36077266 Free PMC article.
References
- Nevins J R. Science. 1992;258:424–429. - PubMed
- Lam E W, La Thangue N B. Curr Opin Cell Biol. 1994;6:859–866. - PubMed
- La Thangue N B. Biochem Soc Trans. 1996;24:54–59. - PubMed
- Sidle A, Palaty C, Dirks P, Wiggan O, Kiess M, Gill R M, Wong A K, Hamel P A. Crit Rev Biochem Mol Biol. 1996;31:237–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous