Purification and characterization of laccase from Chaetomium thermophilium and its role in humification - PubMed (original) (raw)
Purification and characterization of laccase from Chaetomium thermophilium and its role in humification
B Chefetz et al. Appl Environ Microbiol. 1998 Sep.
Abstract
Chaetomium thermophilium was isolated from composting municipal solid waste during the thermophilic stage of the process. C. thermophilium, a cellulolytic fungus, exhibited laccase activity when it was grown at 45 degreesC both in solid media and in liquid media. Laccase activity reached a peak after 24 h in liquid shake culture. Laccase was purified by ultrafiltration, anion-exchange chromatography, and affinity chromatography. The purified enzyme was identified as a glycoprotein with a molecular mass of 77 kDa and an isoelectric point of 5.1. The laccase was stable for 1 h at 70 degreesC and had half-lives of 24 and 12 h at 40 and 50 degreesC, respectively. The enzyme was stable at pH 5 to 10, and the optimum pH for enzyme activity was 6. The purified laccase efficiently catalyzed a wide range of phenolic substrates but not tyrosine. The highest levels of affinity were the levels of affinity to syringaldazine and hydroxyquinone. The UV-visible light spectrum of the purified laccase had a peak at 604 nm (i.e., Cu type I), and the activity was strongly inhibited by Cu-chelating agents. When the hydrophobic acid fraction (the humic fraction of the water-soluble organic matter obtained from municipal solid waste compost) was added to a reaction assay mixture containing laccase and guaiacol, polymerization took place and a soluble polymer was formed. C. thermophilium laccase, which is produced during the thermophilic stage of composting, can remain active for a long period of time at high temperatures and alkaline pH values, and we suggest that this enzyme is involved in the humification process during composting.
Figures
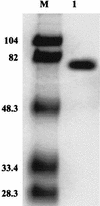
FIG. 1
Coomassie blue-stained PAGE gel containing purified C. thermophilium laccase. Lane M contained standard molecular mass protein markers, including phosphorylase b (molecular mass, 104 kDa), bovine serum albumin, (82 kDa), ovalbumin (48.3 kDa), carbonic anhydrase (33.4 kDa), and soybean trypsin inhibitor (28.3 kDa). Lane 1 contained 10 μg of purified C. thermophilium laccase.
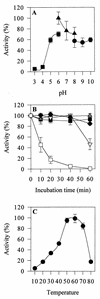
FIG. 2
Effect of pH and temperature on purified C. thermophilium laccase. (A) Enzyme stability after preincubation at various pH values in citrate buffer (■), phosphate buffer (▴), and Tris buffer (•). (B) Enzyme stability after preincubation at 40°C (■), 50°C (▴), 60°C (▾), 70°C (⧫), and 80°C (□). (C) Enzyme activity at various temperatures.
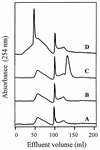
FIG. 3
Fast protein liquid gel filtration chromatography profiles of polymerization assay mixtures containing the HoA fraction (line A), HoA and laccase (line B), HoA and guaiacol (line C), and HoA, guaiacol, and laccase (line D).
Similar articles
- Purification, molecular characterization and reactivity with aromatic compounds of a laccase from basidiomycete Trametes sp. strain AH28-2.
Xiao YZ, Tu XM, Wang J, Zhang M, Cheng Q, Zeng WY, Shi YY. Xiao YZ, et al. Appl Microbiol Biotechnol. 2003 Feb;60(6):700-7. doi: 10.1007/s00253-002-1169-3. Epub 2002 Dec 18. Appl Microbiol Biotechnol. 2003. PMID: 12664149 - Purification and characterisation of a novel laccase from the ascomycete Melanocarpus albomyces.
Kiiskinen LL, Viikari L, Kruus K. Kiiskinen LL, et al. Appl Microbiol Biotechnol. 2002 Jul;59(2-3):198-204. doi: 10.1007/s00253-002-1012-x. Epub 2002 May 7. Appl Microbiol Biotechnol. 2002. PMID: 12111146 - Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium.
Marques De Souza CG, Peralta RM. Marques De Souza CG, et al. J Basic Microbiol. 2003;43(4):278-86. doi: 10.1002/jobm.200390031. J Basic Microbiol. 2003. PMID: 12872309 - Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus.
Shin KS, Lee YJ. Shin KS, et al. Arch Biochem Biophys. 2000 Dec 1;384(1):109-15. doi: 10.1006/abbi.2000.2083. Arch Biochem Biophys. 2000. PMID: 11147821 - Insights into the Applications of Extracellular Laccase-Aided Humification in Livestock Manure Composting.
Li S, Sun K, Latif A, Si Y, Gao Y, Huang Q. Li S, et al. Environ Sci Technol. 2022 Jun 21;56(12):7412-7425. doi: 10.1021/acs.est.1c08042. Epub 2022 May 31. Environ Sci Technol. 2022. PMID: 35638921 Review.
Cited by
- Antifungal activity of resveratrol against Botrytis cinerea is improved using 2-furyl derivatives.
Caruso F, Mendoza L, Castro P, Cotoras M, Aguirre M, Matsuhiro B, Isaacs M, Rossi M, Viglianti A, Antonioletti R. Caruso F, et al. PLoS One. 2011;6(10):e25421. doi: 10.1371/journal.pone.0025421. Epub 2011 Oct 11. PLoS One. 2011. PMID: 22022392 Free PMC article. - Laccase: addressing the ambivalence associated with the calculation of enzyme activity.
Agrawal K, Verma P. Agrawal K, et al. 3 Biotech. 2019 Oct;9(10):365. doi: 10.1007/s13205-019-1895-1. Epub 2019 Sep 21. 3 Biotech. 2019. PMID: 31588389 Free PMC article. - Diversity of laccase-coding genes in Fusarium oxysporum genomes.
Kwiatos N, Ryngajłło M, Bielecki S. Kwiatos N, et al. Front Microbiol. 2015 Sep 10;6:933. doi: 10.3389/fmicb.2015.00933. eCollection 2015. Front Microbiol. 2015. PMID: 26441870 Free PMC article. - Thermophilic fungi: their physiology and enzymes.
Maheshwari R, Bharadwaj G, Bhat MK. Maheshwari R, et al. Microbiol Mol Biol Rev. 2000 Sep;64(3):461-88. doi: 10.1128/MMBR.64.3.461-488.2000. Microbiol Mol Biol Rev. 2000. PMID: 10974122 Free PMC article. Review. - Production, characterization and Congo red dye decolourizing efficiency of a laccase from Pleurotus ostreatus MTCC 142 cultivated on co-substrates of paddy straw and corn husk.
Das A, Bhattacharya S, Panchanan G, Navya BS, Nambiar P. Das A, et al. J Genet Eng Biotechnol. 2016 Dec;14(2):281-288. doi: 10.1016/j.jgeb.2016.09.007. Epub 2016 Oct 26. J Genet Eng Biotechnol. 2016. PMID: 30647626 Free PMC article.
References
- Adani F, Genevini P L, Tambone F. A new index of organic matter stability. Compost Sci Utiliz. 1995;3:25–37.
- Bavendamm W. Uber das Vorkommen und den Nachweis von Oxydasen bei holzzerstoerenden Pilzen. Z Pflanzenkr Pflanzenschutz. 1928;38:257–276.
- Bollag J M, Liu S Y, Minard R D. Enzymatic oligomerization of vanillic acid. Soil Biol Biochem. 1982;14:157–163.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources