Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea - PubMed (original) (raw)
Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea
J A Brock et al. J Physiol. 1998.
Abstract
1. Extracellular recording techniques have been used to study nerve impulses in single sensory nerve terminals in guinea-pig cornea isolated in vitro. 2. Nerve impulses occurred spontaneously and were evoked by electrical stimulation of the ciliary nerves. 3. The nerve impulses were identified as originating in polymodal receptors, mechano-receptors or 'cold' receptors. All three types are believed to be nociceptors. 4. Tetrodotoxin (TTX, 1 microM) blocked nerve impulses evoked by electrical stimulation of the ciliary nerves. However, ongoing and/or naturally evoked nerve impulses persisted in the presence of TTX in all three types of receptors. Lignocaine (lidocaine; 1 mM) blocked all electrical activity. 5. TTX-resistant sodium channels therefore play a major role in generating the action potentials that signal pain to the brain.
Figures
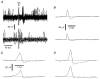
Figure 1. Recording from the corneal epithelium
A, schematic diagram of recording set-up and photomicrograph showing the location of the recording electrode (scale bar, 1 mm). B, a single nerve terminal impulse (NTI) evoked by stimulation of the ciliary nerves. The upper part shows 50 overlaid traces recorded during a train of stimuli at 1 Hz and the lower part shows the average of these traces (SA, stimulation artefact). C, frequency distribution of conduction velocities for all single NTIs recorded. D, confocal micrograph of nerve terminals in the guinea-pig cornea. Most nerve terminals approach the surface of the epithelium at right angles and appear as single dots. On average, 2 terminals lie beneath the opening of a 50 μm pipette (circle).
Figure 2. Spontaneous and evoked NTIs
A, upper part shows overlaid traces in which electrical stimulation of the ciliary nerves evoked a stimulus locked NTI and lower part shows traces in which the occurrence of a spontaneous NTI just before or after the stimulus artefact caused failure of the electrically evoked NTI (SA, stimulation artefact). B, averaged evoked (upper) and spontaneous (lower) NTIs recorded in the same attachment as in A. C and D, averages of electrically evoked (upper traces) and spontaneously occurring (lower traces) NTIs recorded from a mechano-nociceptor (C) and a polymodal receptor (D). The scale bars in C also apply in D.
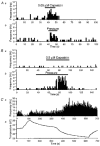
Figure 4. Effects of TTX (1 μm for 30 min)
A-D, averaged electrically evoked (A and C) and spontaneously occurring (B and D) NTIs recorded before (thin line) and in the presence of TTX (thick line) from a mechano-nociceptor (A and B) and a polymodal receptor (C and D). E and F, the effects of capsaicin (0.1 μ
m
) on the frequency of occurrence of NTIs recorded from a polymodal receptor before (E) and during (F) application of TTX. G and H, the effects of temperature changes (upper curve) on the frequency of occurrence of NTIs recorded from a cold-sensitive receptor before (G) and during (H) application of TTX. Histograms of ongoing activity have bin widths of 1 s.
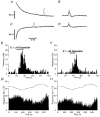
Figure 3. Identification of subtypes of nociceptor
A and B, the effects of capsaicin (Aa and Ba) and mechanical stimulation (Ab and Bb) on the frequency of NTIs in a polymodal receptor (A) and a mechano-nociceptor (B). Ca, the effects of temperature on the frequency of NTIs in a cold-sensitive receptor. Cb, organ bath temperature during the period of recording shown in Ca. In A, B and C the abscissa scale in panel b also applies in panel a. Histograms of ongoing activity have bin widths of 1 s.
Similar articles
- Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea.
Brock JA, Pianova S, Belmonte C. Brock JA, et al. J Physiol. 2001 Jun 1;533(Pt 2):493-501. doi: 10.1111/j.1469-7793.2001.0493a.x. J Physiol. 2001. PMID: 11389207 Free PMC article. - The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea.
Carr RW, Pianova S, Brock JA. Carr RW, et al. J Gen Physiol. 2002 Sep;120(3):395-405. doi: 10.1085/jgp.20028628. J Gen Physiol. 2002. PMID: 12198093 Free PMC article. - Axon reflexes evoked by transient receptor potential vanilloid 1 activation are mediated by tetrodotoxin-resistant voltage-gated Na+ channels in intestinal afferent nerves.
Miranda-Morales M, Ochoa-Cortes F, Stern E, Lomax AE, Vanner S. Miranda-Morales M, et al. J Pharmacol Exp Ther. 2010 Aug;334(2):566-75. doi: 10.1124/jpet.110.165969. Epub 2010 May 3. J Pharmacol Exp Ther. 2010. PMID: 20439439 - Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis.
Acosta CM, Luna C, Quirce S, Belmonte C, Gallar J. Acosta CM, et al. Pain. 2013 Nov;154(11):2353-2362. doi: 10.1016/j.pain.2013.07.012. Epub 2013 Jul 16. Pain. 2013. PMID: 23867735 - Capsaicin-sensitive sensory nerve terminals with local and systemic efferent functions: facts and scopes of an unorthodox neuroregulatory mechanism.
Szolcsányi J. Szolcsányi J. Prog Brain Res. 1996;113:343-59. doi: 10.1016/s0079-6123(08)61097-3. Prog Brain Res. 1996. PMID: 9009744 Review. No abstract available.
Cited by
- Neuropathic pain develops normally in mice lacking both Na(v)1.7 and Na(v)1.8.
Nassar MA, Levato A, Stirling LC, Wood JN. Nassar MA, et al. Mol Pain. 2005 Aug 22;1:24. doi: 10.1186/1744-8069-1-24. Mol Pain. 2005. PMID: 16111501 Free PMC article. - Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones.
Baker MD. Baker MD. J Physiol. 2005 Sep 15;567(Pt 3):851-67. doi: 10.1113/jphysiol.2005.089771. Epub 2005 Jul 7. J Physiol. 2005. PMID: 16002450 Free PMC article. - Roles of Epithelial and Mesenchymal TRP Channels in Mediating Inflammatory Fibrosis.
Okada Y, Sumioka T, Reinach PS, Miyajima M, Saika S. Okada Y, et al. Front Immunol. 2022 Jan 4;12:731674. doi: 10.3389/fimmu.2021.731674. eCollection 2021. Front Immunol. 2022. PMID: 35058918 Free PMC article. Review. - Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease.
Kovács I, Luna C, Quirce S, Mizerska K, Callejo G, Riestra A, Fernández-Sánchez L, Meseguer VM, Cuenca N, Merayo-Lloves J, Acosta MC, Gasull X, Belmonte C, Gallar J. Kovács I, et al. Pain. 2016 Feb;157(2):399-417. doi: 10.1097/j.pain.0000000000000455. Pain. 2016. PMID: 26675826 Free PMC article. - Attenuation of autonomic reflexes by A803467 may not be solely caused by blockade of NaV 1.8 channels.
Stone AJ, Kim JS, Yamauchi K, Ruiz-Velasco V, Kaufman MP. Stone AJ, et al. Neurosci Lett. 2013 May 24;543:177-82. doi: 10.1016/j.neulet.2013.03.015. Epub 2013 Mar 21. Neurosci Lett. 2013. PMID: 23523647 Free PMC article.
References
- Belmonte C, Garcia-Hirschfeld J, Gallar J. Neurobiology of ocular pain. Progress in Retinal and Eye Research. 1997;16:117–156.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources