Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways - PubMed (original) (raw)
Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways
D Vercammen et al. J Exp Med. 1998.
Abstract
Murine L929 fibrosarcoma cells were transfected with the human Fas (APO-1/CD95) receptor, and the role of various caspases in Fas-mediated cell death was assessed. Proteolytic activation of procaspase-3 and -7 was shown by Western analysis. Acetyl-Tyr-Val-Ala-Asp-chloromethylketone and benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone++ +, tetrapeptide inhibitors of caspase-1- and caspase-3-like proteases, respectively, failed to block Fas-induced apoptosis. Unexpectedly, the broad-spectrum caspase inhibitors benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone and benzyloxycarbonyl-Asp(OMe)-fluoromethylketone rendered the cells even more sensitive to Fas-mediated cell death, as measured after 18 h incubation. However, when the process was followed microscopically, it became clear that anti-Fas-induced apoptosis of Fas-transfected L929 cells was blocked during the first 3 h, and subsequently the cells died by necrosis. As in tumor necrosis factor (TNF)-induced necrosis, Fas treatment led to accumulation of reactive oxygen radicals, and Fas-mediated necrosis was inhibited by the oxygen radical scavenger butylated hydroxyanisole. However, in contrast to TNF, anti-Fas did not activate the nuclear factor kappaB under these necrotic conditions. These results demonstrate the existence of two different pathways originating from the Fas receptor, one rapidly leading to apoptosis, and, if this apoptotic pathway is blocked by caspase inhibitors, a second directing the cells to necrosis and involving oxygen radical production.
Figures
Figure 1
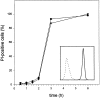
Rapid induction of apoptosis in L929hFas cells. Cells were treated with 500 ng/ml of agonistic anti-Fas mAb in the absence (circles) or presence (triangles) of 1 μg/ml actinomycin D. The fraction of PI-positive cells was determined in a time-course flow fluorocytometric experiment. Inset, Flow fluorocytometric analysis of Fas expression on Fas-transfected cells (solid line) and mock-transfected cells (broken line).
Figure 2
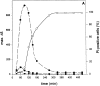
Determination of caspase activity in whole cell lysates of L929hFas cells. Cells were treated with 500 ng/ml of anti-Fas Ab (A) or 10,000 IU/ml of TNF (B) in the absence of actinomycin D. The cleavage activity, expressed as maximal fluorescence increase (max. Δfl.), on Ac-DEVD-amc (filled circles), Ac-YVAD-amc (triangles), and zVAD-afc (squares) was measured as described in Materials and Methods. Before lysis, the percentage of PI-positive cells was determined by flow fluorocytometric analysis (open circles).
Figure 2
Determination of caspase activity in whole cell lysates of L929hFas cells. Cells were treated with 500 ng/ml of anti-Fas Ab (A) or 10,000 IU/ml of TNF (B) in the absence of actinomycin D. The cleavage activity, expressed as maximal fluorescence increase (max. Δfl.), on Ac-DEVD-amc (filled circles), Ac-YVAD-amc (triangles), and zVAD-afc (squares) was measured as described in Materials and Methods. Before lysis, the percentage of PI-positive cells was determined by flow fluorocytometric analysis (open circles).
Figure 3
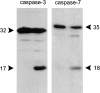
Western blot analysis of caspases activated in L929hFas cells after anti-Fas treatment. Cells were treated with anti-Fas Ab for 30 min or 2 h, and whole cell lysates were subjected to PAGE. After blotting, cleavage products of different procaspases were detected with polyclonal rabbit antisera against the indicated caspases. Procaspase-3 and -7 (32 and 35 kD, respectively) are processed to a large subunit (17 and 18 kD, respectively) and a small subunit (no immunoreactivity). Caspase-1, -2, -6, -11, and -12 fragments were also analyzed (in an identical experiment), but no cleavage was detectable.
Figure 4
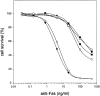
Effect on Fas-mediated cell death of oligopeptide caspase inhibitors in an 18-h assay. 2 h before anti-Fas treatment, 100 μM Ac-YVAD-cmk (filled circles), 100 μM zDEVD-fmk (open circles), 25 μM zVAD-fmk (filled triangles), 25 μM zD-fmk (open triangles), 100 μM zAAD-cmk (filled squares), or medium (open squares) was added. Cell survival was determined by staining with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide as described in Materials and Methods.
Figure 5
Fas-mediated cell death in the absence or presence of zVAD-fmk. Cells were untreated or pretreated with 25 μM zVAD-fmk for 2 h, and incubated in the presence of 500 ng/ml anti-Fas for the indicated times. As a control for necrotic cell death, cells were exposed to 10,000 IU/ml TNF.
Figure 6
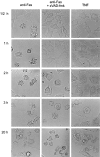
Nuclear morphology and hypoploidy of Fas-mediated cell death. L929hFas cells were preincubated either without (A and D) or with 25 μM zVAD-fmk (B and E) for 2 h, and treated with 5,000 IU/ml TNF (A and B) or 500 ng/ml anti-Fas (C and D) for another 3 h. PI was added, and nuclear morphology was analyzed by confocal microscopy. Necrotic nuclei are PI-positive, but retain a normal structural appearance; apoptotic nuclei are characterized by strong condensation of chromatin. C and F show the fraction of hypoploid cell fragments measured as a function of time. Cells were preincubated without (open circles) or with (filled circles) 25 μM zVAD-fmk, and treated with 500 IU/ml TNF (C) or 500 ng/ml anti-Fas (F).
Figure 7
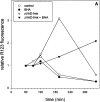
Fas-mediated cell death in the presence of zVAD-fmk is accompanied by oxygen radical production. L929hFas cells were untreated or pretreated with 25 μM zVAD-fmk for 2 h, and incubated with 500 ng/ml anti-Fas or with anti-Fas and BHA. Both the oxygen radical production (A) and the percentage of PI-positive cells (B) were determined under the same conditions.
Figure 7
Fas-mediated cell death in the presence of zVAD-fmk is accompanied by oxygen radical production. L929hFas cells were untreated or pretreated with 25 μM zVAD-fmk for 2 h, and incubated with 500 ng/ml anti-Fas or with anti-Fas and BHA. Both the oxygen radical production (A) and the percentage of PI-positive cells (B) were determined under the same conditions.
Figure 8
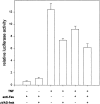
Fas-mediated necrosis is not accompanied by activation of NF-κB. L929hFas cells stably transfected with a luciferase reporter gene were treated for 2 h with 500 ng/ml anti-Fas or 10,000 IU/ml TNF without or with 25 μM zVAD-fmk (2 h pretreatment). NF-κB activity was determined by measuring NF-κB–driven luciferase activity.
Figure 9
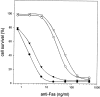
Fas-induced necrosis is not mediated by endogenous TNFRs. L929hFas cells (circles) and TNF-resistant L929r1.hFas cells (triangles) were subjected to treatment with anti-Fas alone (open symbols) or pretreated with 25 μM zVAD-fmk (filled symbols). Cell survival was determined after 18 h incubation by staining with 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide as described in Materials and Methods.
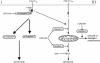
Figure 10
Schematic overview of postulated mechanisms for Fas- mediated cell death. In the absence of inhibitors, the procaspase activation cascade is triggered, including caspases-3 and -7, and the cells die very rapidly by apoptosis (type I). However, when signaling to apoptosis is blocked by caspase inhibitors, secondary signaling components, initiated by still unknown Fas- or TNFR-1–associated components, induce the mitochondria to excessive oxygen radical production (type III). The mechanism of this enhanced oxygen radical production by the addition of zVAD-fmk is still unclear. One may consider three levels at which a putative caspase-X might operate: (a) inhibition of signaling to the mitochondria; (b) inhibition of reactive oxygen intermediates (ROI) scavenging; and (c) removal of damaged mitochondria (reference 27).
Similar articles
- Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor.
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Vercammen D, et al. J Exp Med. 1998 May 4;187(9):1477-85. doi: 10.1084/jem.187.9.1477. J Exp Med. 1998. PMID: 9565639 Free PMC article. - Essential role of caspase-3 in apoptosis of mouse beta-cells transfected with human Fas.
Yamada K, Ichikawa F, Ishiyama-Shigemoto S, Yuan X, Nonaka K. Yamada K, et al. Diabetes. 1999 Mar;48(3):478-83. doi: 10.2337/diabetes.48.3.478. Diabetes. 1999. PMID: 10078546 - CD95/Fas signaling in T lymphocytes induces the cell cycle control protein p21cip-1/WAF-1, which promotes apoptosis.
Hingorani R, Bi B, Dao T, Bae Y, Matsuzawa A, Crispe IN. Hingorani R, et al. J Immunol. 2000 Apr 15;164(8):4032-6. doi: 10.4049/jimmunol.164.8.4032. J Immunol. 2000. PMID: 10754295 - Intracellular mediators of programmed cell death initiated at the cell surface receptor Fas.
Condo I, Testi R. Condo I, et al. Transpl Int. 2000;13 Suppl 1:S3-6. doi: 10.1007/s001470050264. Transpl Int. 2000. PMID: 11111951 Review. - Activation-induced cell death.
Budd RC. Budd RC. Curr Opin Immunol. 2001 Jun;13(3):356-62. doi: 10.1016/s0952-7915(00)00227-2. Curr Opin Immunol. 2001. PMID: 11406369 Review.
Cited by
- Advances in the regulatory mechanisms of mTOR in necroptosis.
Xie Y, Zhao G, Lei X, Cui N, Wang H. Xie Y, et al. Front Immunol. 2023 Dec 18;14:1297408. doi: 10.3389/fimmu.2023.1297408. eCollection 2023. Front Immunol. 2023. PMID: 38164133 Free PMC article. Review. - Working with Auditory HEI-OC1 Cells.
Kalinec GM, Park C, Thein P, Kalinec F. Kalinec GM, et al. J Vis Exp. 2016 Sep 3;(115):54425. doi: 10.3791/54425. J Vis Exp. 2016. PMID: 27684094 Free PMC article. - Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms.
Morgan M, Thorburn J, Pandolfi PP, Thorburn A. Morgan M, et al. J Cell Biol. 2002 Jun 10;157(6):975-84. doi: 10.1083/jcb.200204039. Epub 2002 Jun 3. J Cell Biol. 2002. PMID: 12045187 Free PMC article. - Evidence for a Novel, Caspase-8-Independent, Fas Death Domain-Mediated Apoptotic Pathway.
Feng H, Zeng Y, Graner MW, Whitesell L, Katsanis E. Feng H, et al. J Biomed Biotechnol. 2004;2004(1):41-51. doi: 10.1155/S1110724304308041. J Biomed Biotechnol. 2004. PMID: 15123887 Free PMC article. - Mycoplasma alligatoris infection promotes CD95 (FasR) expression and apoptosis of primary cardiac fibroblasts.
Hunt ME, Brown DR. Hunt ME, et al. Clin Diagn Lab Immunol. 2005 Dec;12(12):1370-7. doi: 10.1128/CDLI.12.12.1370-1377.2005. Clin Diagn Lab Immunol. 2005. PMID: 16339059 Free PMC article.
References
- Grooten J, Goossens V, Vanhaesebroeck B, Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor-induced cytotoxicity. Cytokine. 1993;5:546–555. - PubMed
- Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. - PubMed
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
- Thompson C. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous