Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities - PubMed (original) (raw)
Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities
H Peters et al. Genes Dev. 1998.
Abstract
Pax genes have been shown to play important roles in mammalian development and organogenesis. Pax9, a member of this transcription factor family, is expressed in somites, pharyngeal pouches, mesenchyme involved in craniofacial, tooth, and limb development, as well as other sites during mouse embryogenesis. To analyze its function in vivo, we generated Pax9 deficient mice and show that Pax9 is essential for the development of a variety of organs and skeletal elements. Homozygous Pax9-mutant mice die shortly after birth, most likely as a consequence of a cleft secondary palate. They lack a thymus, parathyroid glands, and ultimobranchial bodies, organs which are derived from the pharyngeal pouches. In all limbs, a supernumerary preaxial digit is formed, but the flexor of the hindlimb toes is missing. Furthermore, craniofacial and visceral skeletogenesis is disturbed, and all teeth are absent. In Pax9-deficient embryos tooth development is arrested at the bud stage. At this stage, Pax9 is required for the mesenchymal expression of Bmp4, Msx1, and Lef1, suggesting a role for Pax9 in the establishment of the inductive capacity of the tooth mesenchyme. In summary, our analysis shows that Pax9 is a key regulator during the development of a wide range of organ primordia.
Figures
Figure 1
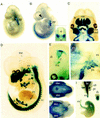
Generation of Pax9_-deficient mice. (A) Structure of wild-type locus, targeting vector, and disrupted Pax9 allele. The ATG–_lacZ–neo cassette was fused to the ATG-containing exon (black box) of Pax9, thereby deleting two thirds of the Pax9 coding sequence including the DNA binding region encoded by the paired box (hatched box). HSV–TK was ligated to the 3′ arm of the targeting vector and was used as a negative selection marker. The 5′ external probe used for genomic Southern blot analysis is shown as a black line. Solid arrowheads indicate the primer located in the ATG-containing exon; open arrowheads indicate allele-specific primers that were used for genotyping. (B) _Bgl_II; (E) _Eco_RV; (N) _Not_I; (RI) _Eco_RI; (S) _Sal_I; (X) _Xma_I; (Xb) _Xba_I. (B) Southern analysis of embryos obtained by heterozygote matings. (C) PCR assay of Pax9lacZ mutant embryos. Yolk sacs of embryos were used for isolation of genomic DNA. PCR was performed with three primers shown in A in a single reaction to distinguish between the wild-type (950 bp) and the transgenic (900 bp) locus. (D) Western blot analysis of Pax9lacZ mutant embryos. The antiserum 281-IV detects a Pax9-specific 38-kD band only in protein extracts prepared from vertebral columns of wild-type and heterozygous embryos (E13.5). (E) Homozygous Pax9lacZ mutant shortly after birth. The mutants are born alive but die within a few hours. During that time, they exhibit gasping respirations and develop a bloated abdomen (arrow).
Figure 2
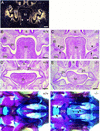
Expression of the Pax9lacZ allele during mouse development revealed by whole-mount X-gal staining of heterozygous Pax9lacZ embryos. (A) Pax9lacZ expression at E9.0 is restricted to the pharyngeal pouches (pp). (B) At E10.5, additional staining is detectable along the body axis and in the facial mesenchyme (arrowhead). The expression in the somites is restricted to the sclerotomes (sc in insert). (C–E) Embryos were cleared with benzylbenzoate/benzylalcohol after X-gal staining. (C) At E12.0, mesenchymes of the nasal processes as well as of the maxillary (mx) and mandibular (md) arches are strongly stained. In addition, expression of Pax9lacZ is seen in the region of the developing lower incisors (in). (D) At E13.5, a number of structures express Pax9lacZ including facial mesenchyme, ear (ea), thymus (th), esophagus (es), forelimb (fl), hindlimb (hl), vertebral column (vc), intercostal mesenchyme (im), and a small domain of the mesencephalon (me). (E) In the mesencephalon, Pax9lacZ expression is restricted to the tegmentum (tg) and the mammillary bodies (ma) at E14.5. (F–I) Pax9lacZ expression at E16.5. (F) Lateral view of the head. The branching ducts of the parotis gland (pa) are Pax9lacZ positive. (G) In the lower jaw, Pax9lacZ expression was found in the tongue (to) and in the region of the developing molars (mo). (H) Similarly, the expression in the upper jaw is strongest in the region of the developing teeth. (I) The derivatives of the foregut endoderm express Pax9lacZ. Expression was detected in the epithelium of the larynx (la), esophagus (es), and forestomach (fs), but also in the thymus (th), parathyroid glands (pt), and ultimobranchial bodies (ub). (cf) Cephalic flexure; (di) diencephalon; (ey) eye; (ln) lateral nasal process; (mn) medial nasal proces; (nt) neural tube.
Figure 3
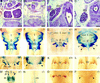
Development of cleft secondary palate in homozygous Pax9lacZ mutant embryos. (A–C) Coronal section of the head at the level of the developing molars at E13.5. (A) Section hybridized with a _Pax9_-specific RNA probe. Pax9 is expressed in the mesenchymes of the nose (n), palatal shelves (ps), and in the mesenchyme underlying the epithelial tooth bud (tb). Note also the expression of Pax9 in the mandibular arch mesenchyme facing the palatal shelves (arrows) and in the lateral tongue (to) mesenchyme (arrowheads). (B) H/E-stained section of a wild-type embryo. (C) H/E-stained section of a homozygous Pax9lacZ embryo. The palatal shelves are present but abnormally broadened. Both lateral indentations of the palatal shelves (arrowheads) as well as the mesenchymal outgrowth (arrows) facing the indentations are absent. (D,E) H/E-stained coronal sections anterior to the developing molars at E14.5. (D) In wild-type embryos, palatal shelves have elevated and are in the process of fusion. (E) In _Pax9_-deficient embryos, bilateral shelve elevation is impaired. (F,G) Skeletal stainings of the secondary palate of newborn mice. Anterior is to the right. (F) In wild-type mice the bony elements of the palatine (p) and the maxillary (m) shelves are almost fused. (G) In homozygous Pax9lacZ mutants both shelves are absent allowing direct view to the vomer (v) and the presphenoid (psp). The borders of the open maxillary and palatine shelves are indicated by arrowheads and arrows, respectively. (mc) Meckels cartilage. Bars, 200 μm.
Figure 4
Arrest of tooth development in homozygous Pax9lacZ mutant embryos. (A–D) H/E-stained coronal sections at the level of the developing molars. (A) At E13.5, mesenchymal cells condense around the epithelial tooth bud. (B) At the same stage, in the homozygous Pax9lacZ mutant embryo, a tooth bud has formed but cellular condensation is reduced (arrow). (C) At E14.5, tooth development has reached the cap stage in the wild-type embryo. (D) In contrast, loose mesenchyme underlying a rudimentary bud is present in the mutant littermate. (E,F) Tooth development in experimental combinations of dental tissues isolated at E13.5. (E) Combinations of wild-type dental mesenchyme and wild-type epithelium yielded well-developed teeth in subrenal grafts. Typical cytodifferentiation was observed including the presence of dentine (dt) secreted by odontoblasts (od), and enamel (en) secreted by ameloblasts (am). (F) A similar result was obtained with normal mesenchyme combined with _Pax9_-deficient dental epithelium. In contrast, combinations of _Pax9_-deficient mesenchyme and normal epithelium failed to form teeth (data not shown). (G–L) In situ hybridization of mesenchymal markers during molar development of normal (G,I,K) and Pax9 mutant (H,J,L) embryos at E13.5. (G) At E13.5, Bmp4 is expressed in the mesenchyme underlying the epithelial tooth bud. In the absence of Pax9 (H), Bmp4 transcripts are barely detectable. (I) Msx1 expression is strong in the tooth mesenchyme and substantially reduced in the absence of Pax9 (J). (K) In wild-type embryos, both the mesenchyme and the margin of the tooth bud epithelium express Lef1. (L) In the absence of Pax9, the mesenchymal expression of Lef1 is reduced whereas the expression in the epithelium is not affected. For clarity, the borders of the tooth buds are outlined by broken lines in H and L. Bars, 100 μm.
Figure 5
Absence of thymus, parathyroid glands, and ultimobranchial bodies in _Pax9_-deficient mice. (A–D) H/E-stained transverse sections of the neck region at E14.5. (A) Two thymic lobes (th) are present in the wild-type embryo. (B) At the same level, _Pax9_-deficient embryos are completely devoid of the thymus. Thymic rudiments were also not found in other regions of the neck and upper trunk (data not shown). (C) The parathyroid glands (pt) are attached to the thyroid gland (thy) in wild-type embryos and are absent in homozygous Pax9lacZ mutant embryos (D). (E–J,M,N) Ventral view of cleared whole-mount X-gal stainings of the pharyngeal pouches and their derivatives in Pax9lacZ mutant embryos. (E) In heterozygous Pax9lacZ mutant embryos at E10.0, Pax9lacZ is expressed in all four pharyngeal pouches (I–IV). (F) At the same stage, the third and fourth pharyngeal pouches are also present in homozygous Pax9lacZ mutant embryos. (G) The third and fourth pharyngeal pouches have started to separate from the pharynx epithelium at E11.5. (H) In homozygous Pax9lacZ mutants, the third (open arrowheads) and fourth (filled arrowheads) pouches are retarded at E11.5. (I) Around E12.0, individual organ primordia of thymus (th), parathyroid glands (pt), and ultimobranchial bodies (ub) become visible as epithelial buds, which are absent in homozygous Pax9lacZ mutants (arrowheads in J). (M) Pax9lacZ expression is maintained in the primordia of thymus, parathyroid glands, and ultimobranchial bodies of heterozygous Pax9lacZ mutant embryos at E14.5, whereas these organs are absent in homozygous Pax9lacZ mutant embryos (N). (K,L,O,P) Ventral view of the fourth pharyngeal pouches of whole-mount embryos stained with HoxB1-specific antibodies. At E10.0, the epithelium of the fourth pouches expresses HoxB1 in both wild-type (K) and _Pax9_-deficient (L) embryos. (O) At E11.5, HoxB1 expression is continued in the fourth pharyngeal pouches. (P) A weak expression of HoxB1 (arrowheads) is detectable in the epithelial remnant of the fourth pharyngeal pouches in homozygous Pax9lacZ mutant embryos. (es) Esophagus; (tr) trachea. Bars, 200 μm.
Figure 6
Craniofacial abnormalities in homozygous Pax9lacZ mutant mice. (A) H/E-stained section of the base of the developing skull at E13.5. (B) Parallel section to A hybridized with a _Pax9_-specific RNA probe. Expression of Pax9 is found in the mesenchyme surrounding the tympanic bulla (tb). (C,D) Ventral view of the skull stained with alizarin red and alcian blue. Anterior is at the top and the lower jaw has been removed. (C) Skeletal elements of the posterior half of the skull. (D) In homozygous Pax9lacZ mutant mice the processus alaris [(pa) asterisks] is absent and the pterygoid process [(pt) arrowheads] is malformed. Note also the severely malformed tympanic rings (arrows). (E) Weak Pax9lacZ expression is detectable in a heterozygous Pax9lacZ mutant at E14.0 in the mesenchyme surrounding Reicherts cartilage (rc). (F) In most homozygous Pax9lacZ mutants Reicherts cartilage is absent, however, Pax9lacZ expression is found in a region in which it normally develops (arrow). (G–M) Skeletal staining of different skeletal elements of newborn mice. (G,H,I) Lateral view of Reicherts cartilage and middle ear. Anterior is to the right. (G) Normal shape of Reicherts cartilage (rc) in a wild-type newborn mouse. (H) In most cases, Reicherts cartilage is not formed in the absence of Pax9 (arrow). (I) In few homozygous Pax9lacZ mutants, Reicherts cartilage is present but abnormally elongated and fused to the hyoid bone (hb). The arrow points to the ossification center of Reicherts cartilage. Note also that in this specimen the tympanic ring (tr) is not affected. (J) Normal appearance of the lower jaw. (K) In homozygous Pax9lacZ mutants the alveolar ridge [(ar) arrowhead] and the coronoid process [(cp) arrow] are absent. (L) Dorsal view of the laryngeal cartilages of normal mice. (M) In the absence of Pax9 both lesser (open arrowheads) and greater (solid arrowheads) horns of the hyoid bone (hb) are malformed. The thyroid cartilage (tc) of the mutant is broadened and lacks lateral processes normally connecting thyroid and cricoid cartilage [(cc) arrows]. (at) Atlas; (bo) basioccipital bone; (bs) basisphenoid; (ea) ear; (mc) Meckels cartilage. Bars, 200 μm.
Figure 7
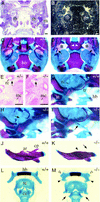
Preaxial digit duplications of hind- and forelimb in _Pax9_-deficient mice. (A,B,C) Pax9lacZ expression during limb development in heterozygous Pax9lacZ mutants. The dorsal view of the left limbs is shown and the anterior–posterior (a,p) axis is indicated. (A) At E11.5, Pax9lacZ expression is seen in an anterior domain of the forelimb (fl) whereas at this stage the expression in the hindlimb (hl) is only weakly detectable (arrowhead). (B) At E12.5 the expression domain is elongated and expression starts in the region of the middle hand/foot. (C) Distinct patches of Pax9lacZ expression appear in the middle hand/foot at E13.5 and the expression in both zeugopods is maintained. (D–G) Skeletal stainings of the left fore- (D,E) and hindlimbs (F,G) of newborn wild-type (D,F) and homozygous Pax9lacZ mutants (E,G). Dorsal views are shown and the digits are numbered. (E) In the forelimb the extra-formed digit (arrow) is not separated from the thumb. (G) In the hindlimb of homozygous Pax9lacZ mutants the duplication of the first digit (arrow) is accompanied by an enlarged cartilaginous area in the region of the anterior metatarsals (asterisk). Note also the abnormal ossification center in the first toe (arrowhead).
Figure 8
Analysis of the hindlimb defects of homozygous Pax9lacZ mutants. (A) Pax9lacZ expression in the fore- (fl) and hindlimb (hl) of a heterozygous Pax9lacZ mutant at E14.0. (B) Pax9lacZ expression in a homozygous Pax9lacZ littermate. Note the strong expression in the developing extra-digits (arrowheads) and the proximally extended expression in the hindlimb (arrow). The level of sections in the panels shown below (C–J) are indicated in A and B. (C–J) Sections of X-gal-stained Pax9 mutant embryos (E14.0) counterstained with nuclear red. (C) At the level of the middle-foot expression of Pax9lacZ is restricted to the mesenchyme between the metatarsals (2–5). (D) A similar expression pattern is seen in the homozygous Pax9lacZ mutant. At the proximal region of the metatarsals the anterior expression domain of Pax9lacZ in the heterozygous mutant (E) is restricted (arrow) to a specific region whereas in the absence of Pax9 (F) the expression is distributed in a wide area of undifferentiated mesenchyme. (G) At the level of the tibiale (tib), the expression of Pax9lacZ covers the region of two developing tendons in the heterozygous Pax9lacZ mutant (t1 and t2, see arrow and magnification in the inset). (H) In the homozygous mutant the tendons are not detectable at this level (arrow). (I) At the distal end of the tibia, a group of three tendons (t1, t2, t3) are detectable. H/E staining confirmed the same arrangement of these tendons in a wild-type embryo (inset). (J) In contrast, in homozygous Pax9lacZ mutants, only one tendon (arrowhead in inset) is detectable in the region of t1 and t2. Note also the ectopic expression of Pax9lacZ in the region where t1 and t2 normally develop. (K–N) Van Gieson staining of transverse sections through the hindlimbs of newborn mice. (K) At the level of the distal end of the tibia, three tendons (t1, t2, t3) are detectable in the wild-type mouse. (L) t2 is missing in the absence of Pax9 (arrow). (M) The musculus flexor digitorum (mfd), which is continuous to t2, is located between the musculus tibialis posterior (mtp) and the musculus flexor hallucis (mfh) and is absent in homozygous Pax9lacZ mutants (arrow in N). (t1) Tendon of musculus tibialis posterior; (t2) Tendon of musculus flexor digitorum; (t3) Tendon of musculus flexor hallucis. Bars, 100 μm.
References
- Baumgartner S, Bopp D, Burri M, Noll M. Structure of two genes at the gooseberry locus related to the paired gene and their spatial expression during embryogenesis. Genes & Dev. 1987;1:1247–1267. - PubMed
- Bopp D, Burri M, Baumgartner S, Frigerio G, Noll M. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell. 1986;47:1033–1040. - PubMed
- Chalepakis G, Stoykova A, Wijnholds J, Tremblay P, Gruss P. Pax: Gene regulators in the developing nervous system. J Neurobiology. 1993;24:1367–1384. - PubMed
- Chen YP, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. - PubMed
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases