Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency - PubMed (original) (raw)
Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency
S Fruehling et al. J Virol. 1998 Oct.
Abstract
Latent membrane protein 2A (LMP2A) of Epstein-Barr virus (EBV) is expressed on the plasma membrane of B lymphocytes latently infected with EBV and blocks B-cell receptor (BCR) signal transduction in EBV-immortalized B cells in vitro. The LMP2A amino-terminal domain that is essential for the LMP2A-mediated block on BCR signal transduction contains eight tyrosine residues. Association of Syk protein tyrosine kinase (PTK) with LMP2A occurs at the two tyrosines of the LMP2A immunoreceptor tyrosine-based activation motif, and it is hypothesized that Lyn PTK associates with the YEEA amino acid motif at LMP2A tyrosine 112 (Y112). To examine the specific association of Lyn PTK to LMP2A, a panel of LMP2A cDNA expression vectors containing LMP2A mutations were transfected into an EBV-negative B-cell line and analyzed for Lyn and LMP2A coimmunoprecipitation. Lyn associates with wild-type LMP2A and other LMP2A mutant constructs, but Lyn association is lost in the LMP2A construct containing a tyrosine (Y)-to-phenylalanine (F) mutation at LMP2A residue Y112 (LMP2AY112F). Next, the LMP2AY112F mutation was recombined into the EBV genome to generate stable lymphoblastoid cell lines (LCLs) transformed with the LMP2AY112F mutant virus. Analysis of BCR-mediated signal transduction in the LMP2AY112F LCLs revealed loss of the LMP2A-mediated block in BCR signal transduction. In addition, LMP2A was not tyrosine phosphorylated in LMP2AY112F LCLs. Together these data indicate the importance of the LMP2A Y112 residue in the ability of LMP2A to block BCR-mediated signal transduction and place the role of this residue and its interaction with Lyn PTK as essential to LMP2A phosphorylation, PTK loading, and down-modulation of PTKs involved in BCR-mediated signal transduction.
Figures
FIG. 1
LMP2A structure and amino acid sequence indicating important motifs of the LMP2A amino-terminal domain. (A) Schematic of the predicted structure of LMP2A in the B-cell plasma membrane. LMP2A contains a 119-amino-acid amino-terminal cytoplasmic domain, 12 hydrophobic transmembrane domains, and a 27-amino-acid cytoplasmic carboxyl domain and is expressed in aggregates in the plasma membranes of latently infected B cells. Numbers denote the locations of the eight tyrosine residues in the LMP2A amino-terminal domain. (B) Amino acid sequence of the LMP2A amino-terminal domain, with the eight tyrosine residues (arrows), four proline-rich motifs (∼∼∼∼∼), and DQSL motif (▪▪▪▪▪) indicated. Each of the eight LMP2A amino-terminal Y residues was mutated to an F residue in pLMP2A cDNA expression vector constructs, including a double Y-to-F mutation at LMP2A residues Y74 and Y85. Four LMP2A deletion mutations, lacking LMP2A residues 21 to 36, 21 to 64, 21 to 85, and 80 to 112, were also incorporated into pLMP2A cDNA expression vector constructs. In addition, multiple LCLs incorporating a Y-to-F mutation at LMP2A Y112 were generated.
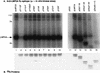
FIG. 2
LMP2A expression and LMP2A phosphoprotein immune complexes in transfected BJABs. Results of in vitro immune complex kinase reactions and 35S-labeled proteins from BJAB cells transfected with wild-type pLMP2A and pLMP2A deletion and point mutants are shown. BJABs cells (107) were transfected with the influenza virus (Flu) HA1 epitope-tagged LMP2A expression vectors, labeled with [35S]methionine, lysed in 1% NP-40, immunoprecipitated (i.p.) with anti-HA1 antibody 12CA5, and captured with protein G-Sepharose, and the immunoprecipitates were divided equally. The first half was separated by SDS-PAGE (7% gel) and transferred to nitrocellulose, and the 35S-labeled LMP2A protein was visualized by autoradiography (B). LMP2A expression was detected in all of the LMP2A-transfected cells (B, lanes 2 to 10 and 12 to 16), while no LMP2A was detected in the control pSG5 transfected cells (B, lane 11). The other half of each immune complex was 32P labeled in vitro by an immune complex kinase reaction and separated by SDS-PAGE (7% gel), and the resulting 32P-labeled proteins were visualized by autoradiography (A) after blocking of the 35S-labeled proteins by two sheets of film. A number of phosphorylated proteins complex with both wild-type and mutant pLMP2A proteins (A, lanes 1 to 10 and 12 to 16), including LMP2A (arrow), 72-kDa Syk, and 53- to 60-kDa Src family PTKs, as well as unidentified proteins of 96, 104, 110, and 112 kDa. The pY112 and p2AΔ80-112 mutants demonstrate a complete reduction of total phosphorylation (A, lanes 2 and 16). No proteins were detected in the control pSG5-transfected cells (A, lane 11). Protein standards are indicated at the left in kilodaltons.
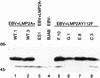
FIG. 3
Lyn binds to Y112 of LMP2A. BJAB cells (107) were transfected with HA1 epitope-tagged LMP2A expression vectors, labeled with [35S]methionine, lysed in 1% NP-40, immunoprecipitated (i.p.) with the anti-HA1 antibody 12CA5, captured with protein G-Sepharose, separated by SDS-PAGE (7% gel), and transferred to nitrocellulose, and the immunoprecipitated LMP2A protein was visualized by autoradiography (B). LMP2A expression within the transfected BJAB cells was detected in all of the LMP2A-transfected cells (B, lanes 23 to 32 and 34 to 38), while no LMP2A was detected in the control pSG5-transfected cells (B, lanes 22 and 33). The membrane was then immunoblotted with a polyclonal Lyn antiserum and incubated with an HRP-conjugated secondary antibody, and proteins were detected by ECL (A). Lyn coimmunoprecipitated with LMP2A in BJAB cells transfected with the wild-type pLMP2A expression vector (A, lanes 4 and 17) and not with control pSG5 vector (A, lanes 3 and 16). Lyn also coimmunoprecipitated with pLMP2A constructs containing tyrosine-to-phenylalanine point mutations at LMP2A tyrosine residues (Y101F, Y7485F, Y85F, Y74F, Y64F, Y60F, Y31F, and Y23F [A, lanes 6 to 13]), as well as pLMP2A deletion mutants that lack LMP2A residues 21 to 36, 21 to 64, and 21 to 85 (A, lanes 18 to 20). However, Lyn did not coimmunoprecipitate with LMP2A in the Y112F point mutant (A, lane 5) or the deletion mutant lacking residues 80 to 112 (lane 21). BJAB and HPB.ALL cellular lysates were included as positive and negative controls for Lyn expression, respectively (A, lanes 1, 2, 14, and 15). Protein standards are indicated at the left in kilodaltons.
FIG. 4
LMP2A expression in EBV+LMP2A+ and EBV+LMP2AY112F LCLs. NP-40 lysates of LCLs (5 × 106 cells/lane) were separated by SDS-PAGE (8% gel), transferred to Immobilon, immunoblotted with anti-LMP2A antiserum 14B7, and incubated with an HRP-conjugated secondary antibody, and proteins were detected by ECL. The levels of LMP2A expression were similar in two wild-type EBV+LMP2A+ LCLs (lanes 1 and 2) and four EBV+LMP2AY112F LCLs (lanes 5 to 8). LMP2A expression was not detected in an EBV+LMP2A− LCL (lane 3) or the EBV− cell line BJAB (lane 4). LCL clone numbers are indicated above each lane, and protein standards are indicated at the left in kilodaltons.
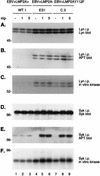
FIG. 5
LMP2A is not phosphorylated in EBV+LMP2AY112F LCLs. LCLs were untreated (−) or treated at 3 × 107 cells per ml with anti-BCR antibodies for the indicated times (1 or 5 min), lysed in NP-40, immunoprecipitated (i.p.) with anti-LMP2A 14B7 antiserum, captured with protein G-Sepharose, separated by duplicate SDS-PAGE (6% gel), and transferred to Immobilon. Parallel immunoblots were probed with anti-LMP2A (14B7-biotin) antiserum (A) or APT antibody PY20 (B), incubated with HRP-conjugated secondary antibodies, and detected by ECL. (A) LMP2A expression levels in two EBV+LMP2A+ LCLs (A, lanes 1 to 6) and two EBV+LMP2AY112F LCLs (A, lanes 10 to 15) were similar in all four LCLs during the time course. No LMP2A was detected in the EBV+LMP2A− LCL included as a negative control (A, lanes 7 to 9). (B) LMP2A was constitutively phosphorylated in two EBV+LMP2A+ LCLs which did not change after BCR cross-linking (B, lanes 1 to 6). In contrast, LMP2A was not phosphorylated in the two EBV+LMP2AY112F LCLs even after BCR cross-linking (B, lanes 10 to 15). No phosphorylated LMP2A was detected in the negative control EBV+LMP2A− LCL (B, lanes 7 to 9). LCL clone numbers are indicated above each group of lanes, and protein standards are indicated at the left in kilodaltons.
FIG. 6
Expression, phosphorylation, and kinase activities of Lyn and Syk in EBV+LMP2A+, EBV+LMP2A−, and EBV+LMP2AY112F LCLs following BCR cross-linking. LCLs were untreated (−) or treated at 5 × 107 cells per ml with anti-BCR antibodies for the indicated times (1 or 5 min) and lysed in 1% NP-40. Lysates were divided equally, immunoprecipitated (i.p.) in parallel with either a Lyn monoclonal antibody (A to C) or a Syk monoclonal antibody (D to F), and captured with protein A-Sepharose beads. One third of each immunoprecipitation was further subjected to an in vitro kinase assay (C and F). All immune complexes were separated in parallel by SDS-PAGE (6% gel). The 32P-labeled products were visualized by autoradiography of the dried gels (C and F). The nonradioactive proteins were transferred to Immobilon and immunoblotted in parallel with monoclonal APT (B and E), anti-Lyn (A), or anti-Syk (D) antibodies, followed by incubation with HRP-conjugated secondary antibodies and ECL detection. (A) The level of p56/p53 Lyn expression did not change following BCR cross-linking in the EBV+LMP2A+, EBV+LMP2A−, and EBV+LMP2AY112F LCLs (A, lanes 1 to 9). (B) Lyn remained constitutively phosphorylated before and after BCR cross-linking in the three LCLs, but the level of p56/p53 Lyn phosphorylation was elevated in the EBV+LMP2A− and EBV+LMP2AY112F LCLs (B, lanes 4 to 9) compared with the barely detectable Lyn phosphorylation in the EBV+LMP2A+ LCL (B, lanes 1 to 3). (C) Lyn remained constitutively active in autophosphorylation assays before and after BCR cross-linking in the three LCLs, but the level of p56/p53 Lyn activity was increased in the EBV+LMP2A− and EBV+LMP2AY112F LCLs (C, lanes 4 to 9) compared with the barely detectable Lyn activity in the EBV+LMP2A+ LCL (C, lanes 1 to 3). (D) The level of 72-kDa Syk expression was similar in the EBV+LMP2A+, EBV+LMP2A−, and EBV+LMP2AY112F LCLs following BCR cross-linking (D, lanes 1 to 9). (E) Syk remained constitutively phosphorylated before and after BCR cross-linking in the EBV+LMP2A+ LCL (E, lanes 1 to 3), while Syk demonstrated no constitutive phosphorylation before BCR cross-linking in the EBV+LMP2A− and EBV+LMP2AY112F LCLs (E, lanes 4 and 7) and became rapidly phosphorylated within 1 min after BCR cross-linking in both the EBV+LMP2A− and EBV+LMP2AY112F LCLs (E, lanes 5 to 6 and 8 to 9). (F) Syk demonstrated a low level of constitutive activity before and after BCR cross-linking in the EBV+LMP2A+ LCL (F, lanes 1 to 3), whereas Syk demonstrated a higher level of constitutive activity that induced to even greater levels following BCR cross-linking in the EBV+LMP2A− and EBV+LMP2AY112F LCLs (F, lanes 4 to 9). LCL clone numbers are indicated above each group of lanes, and protein standards are indicated at the left in kilodaltons.
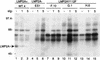
FIG. 7
Tyrosine phosphorylation following BCR cross-linking in EBV+LMP2A+, EBV+LMP2A−, and EBV+LMP2AY112F LCLs. LCLs were untreated (−) or treated at 3 × 107 cells per ml for the indicated times (1 or 5 min) with anti-BCR antibodies, lysed in 1% NP-40, immunoprecipitated with APT antibody PY20, captured with protein A-Sepharose, separated by SDS-PAGE (6% gel), transferred to Immobilon, and immunoblotted with HRP-conjugated APT antibody PY20, and proteins were detected by ECL. There was no induction of tyrosine phosphorylation in an EBV+LMP2A+ LCL (lanes 1 to 3), in contrast to the dramatic increase in phosphorylation in an EBV+LMP2A− LCL (lanes 4 to 6) and three EBV+LMP2AY112F LCLs (lanes 7 to 15). LCL clone numbers are indicated above each group of lanes, and protein standards are indicated at the left in kilodaltons.
FIG. 8
Induction of BZLF1 expression in EBV+LMP2A− and EBV+LMP2AY112F LCLs following BCR cross-linking. LCLs (4 × 106) were untreated (−) or treated (+) with anti-BCR antibodies for 48 h. Whole-cell lysates were separated by SDS-PAGE (8% gel), transferred to Immobilon, immunoblotted with anti-BZLF1 antibody BZ1, and incubated with an HRP-conjugated secondary antibody, and proteins were detected by ECL. In five EBV+LMP2AY112F LCLs, BZLF1 expression was induced after anti-BCR treatment (lanes 8, 10, 12, 14, and 16), similar to the result for two EBV+LMP2A− LCLs, included as positive controls (lanes 4 and 6). However, BZLF1 expression was not induced after anti-BCR treatment in an EBV+LMP2A+ LCL, included as a negative control (lane 2). Prior to BCR cross-linking, BZLF1 expression was not detected in any of the LCLs (lanes 1, 3, 5, 7, 9, 11, 13, and 15). LCL clone numbers are indicated above each pair of lanes, and protein standards are indicated at the left in kilodaltons.
FIG. 9
Model of LMP2A function. (A) The Src family PTK Lyn is recruited to LMP2A possibly by an interaction of the Lyn SH3 domain with the LMP2A proline-rich regions or an interaction of the Lyn unique region (U) with the LMP2A DQSL sequence. WW domain-containing proteins may also be recruited to the LMP2A nonphosphorylated PPPPY motifs. (B) LMP2A becomes phosphorylated at Y112 by the Lyn PTK or another unidentified cellular PTK, possibly the p57 or p60 protein whose phosphorylation is observed in both wild-type and LMP2AY112F LCLs prior to BCR cross-linking. (C) Once Y112 is phosphorylated, the Lyn SH2 domain binds. Following Lyn binding to LMP2A Y112, the remaining LMP2A tyrosines, including the LMP2A ITAM, become phosphorylated. Binding of Lyn to LMP2A and subsequent LMP2A phosphorylation are blocked in the Y112F mutants; thus, the Y112F mutants do not proceed to step C. (D) The presence of other phosphorylated LMP2A motifs allows the binding of the Syk PTK, other SH2-containing proteins, and MAPK to phosphorylated LMP2A. Once bound to LMP2A, their activities are reduced and they are no longer able to participate in BCR signal transduction. Binding of Syk to LMP2A is blocked in the LMP2A ITAM (Y74F and Y85F) mutants, although LMP2A still demonstrates the phosphorylation of other tyrosines. The LMP2A ITAM mutants do not proceed fully to step D, due to the loss of Syk binding to LMP2A.
Similar articles
- Tyrosines 60, 64, and 101 of Epstein-Barr virus LMP2A are not essential for blocking B cell signal transduction.
Swart R, Fruehling S, Longnecker R. Swart R, et al. Virology. 1999 Oct 25;263(2):485-95. doi: 10.1006/viro.1999.9964. Virology. 1999. PMID: 10544120 - The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction.
Fruehling S, Longnecker R. Fruehling S, et al. Virology. 1997 Sep 1;235(2):241-51. doi: 10.1006/viro.1997.8690. Virology. 1997. PMID: 9281504 - PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins.
Ikeda M, Ikeda A, Longnecker R. Ikeda M, et al. J Virol. 2001 Jun;75(12):5711-8. doi: 10.1128/JVI.75.12.5711-5718.2001. J Virol. 2001. PMID: 11356981 Free PMC article. - Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction.
Miller CL, Lee JH, Kieff E, Burkhardt AL, Bolen JB, Longnecker R. Miller CL, et al. Infect Agents Dis. 1994 Apr-Jun;3(2-3):128-36. Infect Agents Dis. 1994. PMID: 7812651 Review. - The LMP2A signalosome--a therapeutic target for Epstein-Barr virus latency and associated disease.
Portis T, Cooper L, Dennis P, Longnecker R. Portis T, et al. Front Biosci. 2002 Feb 1;7:d414-26. doi: 10.2741/portis. Front Biosci. 2002. PMID: 11815296 Review.
Cited by
- Molecular Interactions between Two LMP2A PY Motifs of EBV and WW Domains of E3 Ubiquitin Ligase AIP4.
Seo MD, Seok SH, Kim JH, Choi JW, Park SJ, Lee BJ. Seo MD, et al. Life (Basel). 2021 Apr 22;11(5):379. doi: 10.3390/life11050379. Life (Basel). 2021. PMID: 33922228 Free PMC article. - Epstein-Barr virus in Burkitt's lymphoma: a role for latent membrane protein 2A.
Bieging KT, Swanson-Mungerson M, Amick AC, Longnecker R. Bieging KT, et al. Cell Cycle. 2010 Mar 1;9(5):901-8. doi: 10.4161/cc.9.5.10840. Epub 2010 Mar 3. Cell Cycle. 2010. PMID: 20160479 Free PMC article. - C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal.
Matskova L, Ernberg I, Pawson T, Winberg G. Matskova L, et al. J Virol. 2001 Nov;75(22):10941-9. doi: 10.1128/JVI.75.22.10941-10949.2001. J Virol. 2001. PMID: 11602734 Free PMC article. - Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells.
Morrison JA, Klingelhutz AJ, Raab-Traub N. Morrison JA, et al. J Virol. 2003 Nov;77(22):12276-84. doi: 10.1128/jvi.77.22.12276-12284.2003. J Virol. 2003. PMID: 14581564 Free PMC article. - EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway.
Anderson LJ, Longnecker R. Anderson LJ, et al. J Gen Virol. 2008 Jul;89(Pt 7):1563-1568. doi: 10.1099/vir.0.2008/001461-0. J Gen Virol. 2008. PMID: 18559925 Free PMC article.
References
- Bolen J B. Nonreceptor tyrosine protein kinases. Oncogene. 1993;8:2025–2031. - PubMed
- Cambier J C. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol Today. 1995;16:110. - PubMed
- Cambier J C, Pleiman C M, Clark M R. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous