Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression - PubMed (original) (raw)
Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression
O Devergne et al. J Virol. 1998 Oct.
Abstract
In this study, we investigated the induction of cellular gene expression by the Epstein-Barr Virus (EBV) latent membrane protein 1 (LMP1). Previously, LMP1 was shown to induce the expression of ICAM-1, LFA-3, CD40, and EBI3 in EBV-negative Burkitt lymphoma (BL) cells and of the epidermal growth factor receptor (EGF-R) in epithelial cells. We now show that LMP1 expression also increased Fas and tumor necrosis factor receptor-associated factor 1 (TRAF1) in BL cells. LMP1 mediates NF-kappaB activation via two independent domains located in its C-terminal cytoplasmic tail, a TRAF-interacting site that associates with TRAF1, -2, -3, and -5 through a PXQXT/S core motif and a TRADD-interacting site. In EBV-transformed B cells or transiently transfected BL cells, significant amounts of TRAF1, -2, -3, and -5 are associated with LMP1. In epithelial cells, very little TRAF1 is expressed, and only TRAF2, -3, and -5, are significantly complexed with LMP1. The importance of TRAF binding to the PXQXT/S motif in LMP1-mediated gene induction was studied by using an LMP1 mutant that contains alanine point mutations in this motif and fails to associate with TRAFs. This mutant, LMP1(P204A/Q206A), induced 60% of wild-type LMP1 NF-kappaB activation and had approximately 60% of wild-type LMP1 effect on Fas, ICAM-1, CD40, and LFA-3 induction. In contrast, LMP1(P204A/Q206A) was substantially more impaired in TRAF1, EBI3, and EGF-R induction. Thus, TRAF binding to the PXQXT/S motif has a nonessential role in up-regulating Fas, ICAM-1, CD40, and LFA-3 expression and a critical role in up-regulating TRAF1, EBI3, and EGF-R expression. Further, D1 LMP1, an LMP1 mutant that does not aggregate failed to induce TRAF1, EBI3, Fas, ICAM-1, CD40, and LFA-3 expression confirming the essential role for aggregation in LMP1 signaling. Overexpression of a dominant form of IkappaBalpha blocked LMP1-mediated TRAF1, EBI3, Fas, ICAM-1, CD40, and LFA-3 up-regulation, indicating that NF-kappaB is an important component of LMP1-mediated gene induction from both the TRAF- and TRADD-interacting sites.
Figures
FIG. 1
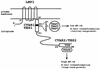
Schematic representation of LMP1. LMP1 consists of a 23-aa N-terminal cytoplasmic domain, six hydrophobic transmembrane domains separated by short reverse turns, and a 200-aa CTD. The two signaling domains, LMP1 aa 187 to 231 (CTAR1/TES1) and aa 352 to 386 (CTAR2/TES2), are represented by empty boxes; the core of the TRAF binding site, aa 201 to 210, is represented by a black box. Residues directly implicated in TRAF or TRADD binding are indicated. Residues marked with an asterisk were mutated to alanine in the LMP1(P204A/Q206A) mutant. D1 LMP1 comprises the last two transmembrane domains and the CTD, i.e., LMP1 aa 129 to 386.
FIG. 2
Effect of the P204A/Q206A mutation on TRAFs binding to LMP1. (A) BJAB cells (107 per transfection) or C33A cells (4 × 106 per transfection) were electroporated with 20 μg of plasmid pSG5 expressing Flag-LMP1 (F-LMP1) (10), 20 (left) or 30 (right) μg of pSG5 Flag-LMP1(P204A/Q206A) (F-LMP PQAA) (10), or 20 μg of pSG5 EBI3-Flag (EBI3-F) (9) as a control. Eighteen hours posttransfection, cells were lysed in 0.5% NP-40 lysis buffer, and cell extracts were submitted to immunoprecipitation (IP) with anti-Flag affinity gel. A fraction of the cell lysate obtained before immunoprecipitation (left, lanes 1 to 3; right, lanes 2 to 4) and total immunoprecipitates (left, lanes 4 to 6; right, lanes 5 to 7) were analyzed by SDS-PAGE on an 8% gel and subjected to Western blot analysis with rabbit polyclonal antibodies recognizing TRAF1 (S-19 or H-132), TRAF2 (C-20), or TRAF3 (H-122), with anti-Flag antibody M5 (left), or with anti-LMP1 monoclonal antibody S12 (right). LMP1 and LMP1(P204A/Q206A) display similar solubilities under the conditions of extraction used here, so that the amount of LMP1 protein observed in the cell lysate fraction is representative of the whole LMP1 protein content (data not shown). Cell extract from 5 × 105 IB4 cells was used as a control for TRAF1 detection (right, lane 1). The positions of the TRAF1 doublet and of TRAF2, TRAF3, and LMP1 are indicated. (B) NP-40 cell extracts from 80 × 106 cells from a recombinant LCL expressing an N-terminal Flag-tagged LMP1 protein (F-LMP1) or of a control IB4 LCL expressing non-Flag-tagged LMP1 (LMP1) were subjected to immunoprecipitation with M2 anti-Flag affinity gel. A fraction of the cell lysates obtained before (input; lanes 1 and 2) or after (unbound; lanes 3 and 4) immunoprecipitation, or of the unsoluble fraction (unsol; lanes 5 and 6), and the total immunoprecipitates (lanes 7 and 8) were analyzed by SDS-PAGE on a 10% gel and subjected to Western blot analysis with rabbit polyclonal antibodies recognizing TRAF2 (C-20), TRAF3 (H-122), and TRAF4 (N-16) and with goat polyclonal antibodies recognizing TRAF5 (C-19). The positions of the TRAFs are marked; immunoglobulin heavy chain is indicated by an asterisk. Western blotting with anti-Flag antibody Ms showed that more than 50% of LMP1 was solubilized and precipitated (data not shown). (C) BJAB cells (107 per transfection) or C33A cells (5 × 106 per transfection) were electroporated with 25 μg of pSG5 Flag-LMP1 (F-LMP1), 35 μg of pSG5 Flag-LMP1(P204A/Q206A) (F-LMP PQAA), or 25 μg of pSG5 EBI3-Flag (EBI3-F) as a control. Eighteen hours posttransfection, cells were lysed in 0.5% NP-40 lysis buffer, and cell extracts were submitted to immunoprecipitation with anti-Flag affinity gel. Immunoprecipitates were analyzed by SDS-PAGE on a 10% gel and subjected to Western blot analysis with rabbit polyclonal antibodies recognizing TRAF5 (H-257) or with anti-Flag antibody M5. The positions of TRAF5 and LMP1 are indicated.
FIG. 3
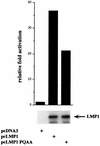
NF-κB induction by LMP1 and LMP1(P204A/Q206A) in 293 cells. 293 cells (5 × 105) were transfected with 350 ng of the luciferase reporter driven by three NF-κB binding sites from the major histocompatibility complex class I promoter, 350 ng of pGK-β-galactosidase, and 375 ng of pcDNA-based expression plasmids. Data are presented as luciferase activity normalized to β-galactosidase activity and adjusted to 1 for pcDNA3 control-transfected cells. 293 lysates were normalized for protein content and analyzed for LMP1 expression by immunoblot analysis using anti-LMP1 monoclonal antibody S12.
FIG. 4
Effect of the P204A/Q206A mutation on EGF-R induction in C33A cells. Whole-cell extracts from four independent C33A clones stably transfected with either pcDNA3 (vector; lanes 1, 2, 7, and 8), pcDNA3 expressing wild-type LMP1 (WT; lanes 3, 4, 9, and 10), or LMP1(P204A/Q206A) (PQAA; lanes 5, 6, 11, and 12) were immunoblotted with rabbit polyclonal antibodies recognizing EGF-R or with anti-LMP1 monoclonal antibody S12. Numbers at the left show positions of standard molecular weight proteins (in thousands). The intensity of the 120-kDa cross-reactive protein observed with EGF-R antibodies paralleled that of the Ponceau staining and is indicative of the total amount of protein loaded. Due to the very low amount of LMP1 protein present in lane 4, the S12 blot shown on the left was overexposed compared to the blot shown on the right. Immunoblot analysis was performed after over 40 days of selection.
FIG. 5
Immunoblot analysis of TRAF1 and EBI3 induction by LMP1 mutants in transfected BJAB cells. (A) BJAB cells (107 per transfection) were transiently transfected with increasing amounts (10, 20, and 40 μg) of pSG5 vector expressing wild-type LMP1 (WT) or LMP1(P204A/Q206A) (PQAA) or with 20 μg of pSG5 vector expressing D1 LMP1 (D1) (42). Cells extracts (106 cells per lane) obtained 20 h posttransfection were immunoblotted with a rabbit polyclonal antibody recognizing TRAF1 (H-132), with affinity-purified rabbit polyclonal anti-EBI3 antibodies, or with anti-LMP1 monoclonal antibody S12. The positions of LMP1 and its derivatives, of the TRAF1 doublet, and of EBI3 are indicated on the right. Numbers at the left show positions of standard molecular weight proteins (in thousands). Ponceau S staining of the blot showed equal total protein loading per lane (data not shown). Expression of a higher amount of D1 LMP1 did not result in significant induction of TRAF1 (data not shown). (B) Cell extracts (106 cells per lane) from independent BJAB clones stably expressing wild-type LMP1 or LMP1(P204A/Q206A) were analyzed by immunoblotting for TRAF1 and EBI3 expression as described above. BJ MTLMP (lanes 1 and 2) are stably transfected BJAB cell lines expressing (+) or not expressing (−) LMP1 under the control of a metallothionein promoter (59); lanes 4 and 5 contain two pcDNA3 vector control-derived BJAB clones; BJ pcLMP (lanes 6 and 7) and BJpcLMP PQAA (lanes 8 and 9) stably express wild-type LMP1 and LMP1(P204A/Q206A), respectively, under the control of a CMV promoter. IB4 (lane 3) is an EBV-transformed LCL. The positions of LMP1, TRAF1 doublet, and EBI3 are indicated on the right. Numbers at the left show positions of standard molecular weight proteins (in thousands). Ponceau S staining of the blot showed equal total protein loading per lane (data not shown).
FIG. 6
FACS analysis of cell surface molecule induction by wild-type and mutant LMP1 in transiently transfected BL41 cells. BL41 cells (5 × 106) were electroporated with pSG5 vector alone or pSG5 vector expressing wild-type LMP1 (WT; 20 μg), LMP1(P204A/Q206A) (PQAA; 30 μg), or D1 LMP1 (D1; 20 μg), together with 3 μg of GFP reporter plasmid. Twenty-two hours posttransfection, one fraction of the cells was stained for ICAM-1, Fas, CD40, and LFA-3 cell surface expression and examined by two-color FACS analysis as indicated in Materials and Methods; a second fraction was analyzed by immunoblotting for LMP1 expression. FL2 fluorescence of GFP-positive cells only is represented (X axis, fluorescence intensity; y axis, cell number). The dotted line corresponds to vector control-transfected cells; the thick line represents LMP1- or LMP1 mutant-transfected cells. To quantify the relative efficiency of wild-type and mutant LMP1 proteins to up-regulate surface expression of these markers, a gate was set so that 5% of the vector control-transfected cells scored positive for the surface marker analyzed. The percentages on each graph corresponds to the fraction of LMP1-expressing cells scoring positive. Data from one representative experiment of three are represented. Wild-type and mutants LMP1 proteins were expressed at similar levels, as assessed by immunoblotting with anti-LMP1 monoclonal antibody S12.
FIG. 7
Effect of IκBα S32AS36A overexpression on LMP1-mediated Fas up-regulation. BL41 cells were transfected with 3 μg of GFP expression vector and the indicated amounts (in micrograms) of pSG5 LMP1 and pCMV4 IκBα S32AS36A. Total DNA transfected was kept constant by addition of vector DNA. (A) Approximately 20 h posttransfection, one fraction of the cells was tested for Fas, ICAM-1, CD40, and LFA-3 surface expression by two-color FACS analysis as described for Fig. 6. Only data for Fas are shown. (B) Whole-cell lysates (5 × 105 cells per lane) were analyzed by immunoblotting for LMP1 expression with anti-LMP1 monoclonal antibody S12.
FIG. 8
Effect of IκBα S32AS36A overexpression on TRAF1 and EBI3 induction by LMP1. BJAB cells (5 × 106 per transfection) were transiently transfected with 3 μg of GFP expression vector and the indicated amounts (in micrograms) of pSG5 LMP1 (A) or pcDNA LMP1 (B) and of pCMV4 IκBα S32AS36A. Total DNA transfected was maintained constant by addition of vector DNA. Cells extracts (5 × 105 cells per lane) obtained 20 to 22 posttransfection were subjected to immunoblot analysis with a rabbit polyclonal antibody recognizing TRAF1 (H-132), with affinity-purified rabbit polyclonal anti-EBI3 antibodies, or with anti-LMP1 monoclonal antibody S12. The positions of LMP1, the TRAF1 doublet, and EBI3 are indicated on the right.
References
- Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, Yagita H, Okumura K, Inoue J, Watanabe T. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J Biol Chem. 1997;272:2042–2045. - PubMed
- Baichwal V R, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of the Epstein-Barr virus. Oncogene. 1988;2:461–467. - PubMed
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. - PubMed
- Behrmann I, Walczak H, Krammer P H. Structure of the human APO-1 gene. Eur J Immunol. 1994;24:3057–3062. - PubMed
- Brodeur S R, Cheng G, Baltimore D, Thorley-Lawson D A. Localisation of the major NF-κB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J Biol Chem. 1997;272:19777–19784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007061/AI/NIAID NIH HHS/United States
- AI 07061-20/AI/NIAID NIH HHS/United States
- R01 CA047006/CA/NCI NIH HHS/United States
- R35 CA047006/CA/NCI NIH HHS/United States
- CA470006/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous