Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo - PubMed (original) (raw)
Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo
J M Lubinski et al. J Virol. 1998 Oct.
Abstract
Many microorganisms encode proteins that interact with molecules involved in host immunity; however, few of these molecules have been proven to promote immune evasion in vivo. Herpes simplex virus type 1 (HSV-1) glycoprotein C (gC) binds complement component C3 and inhibits complement-mediated virus neutralization and lysis of infected cells in vitro. To investigate the importance of the interaction between gC and C3 in vivo, we studied the virulence of a gC-null strain in complement-intact and C3-deficient animals. Using a vaginal infection model in complement-intact guinea pigs, we showed that gC-null virus grows to lower titers and produces less severe vaginitis than wild-type or gC rescued virus, indicating a role for gC in virulence. To determine the importance of complement, studies were performed with C3-deficient guinea pigs; the results demonstrated significant increases in vaginal titers of gC-null virus, while wild-type and gC rescued viruses showed nonsignificant changes in titers. Similar findings were observed for mice where gC null virus produced significantly less disease than gC rescued virus at the skin inoculation site. Proof that C3 is important was provided by studies of C3 knockout mice, where disease scores of gC-null virus were significantly higher than in complement-intact mice. The results indicate that gC-null virus is approximately 100-fold (2 log10) less virulent that wild-type virus in animals and that gC-C3 interactions are involved in pathogenesis.
Figures
FIG. 1
(A) Particle-to-infectivity ratios of wild-type and gC mutant viruses. NS or NS-gCnull virus (106 PFU of each) was tested by Western blotting for viral capsid antigen VP5. Bands were quantified by densitometry. The two viruses had similar PFU/VP5 ratios, which indicates comparable plaquing efficiency. Sizes are indicated in kilodaltons. (B) Attachment kinetics of NS or NS-gCnull virus. [35S]methionine- and [35S]cysteine-labeled cell-free virus was added to HEp-2 cells at 4°C, and at various times bound counts were determined. NS and NS-gCnull have similar binding kinetics.
FIG. 2
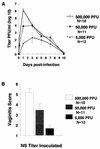
Dose-response studies with wild-type virus NS. (A) NS was inoculated intravaginally at 5 × 105, 5 × 104, or 5 × 103 PFU. Vaginal swabs were taken 4 h and at 1, 3, 5, 7, and 10 days postinfection, and titers were determined. AUC was calculated as a measure of viral load. AUCs were 28.3 at 5 × 105 PFU, 24.6 at 5 × 104 PFU, and 14.3 at 5 × 103. AUC at 5 × 105 PFU is significantly greater than AUC at 5 × 103 PFU (P < 0.01), and AUC at 5 × 104 PFU is significantly greater than AUC at 5 × 103 PFU (P = 0.05). (B) Vaginitis scores at 5 × 105, 5 × 104, and 5 × 103 PFU. Scores at 5 × 105 PFU are significantly greater than scores at 5 × 104 PFU (P = 0.03), and scores at 5 × 104 are significantly greater than scores at 5 × 103 PFU (P < 0.01). Error bars represent ± SEM.
FIG. 3
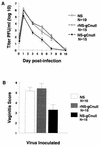
Vaginal titers and vaginitis scores are lower for gC-null virus than for rescued and wild-type strains. (A) Guinea pigs were inoculated with 5 × 105 PFU of NS, rNS-gCnull, or NS-gCnull virus. Vaginal swabs were taken at the indicated times, and titers were measured. AUCs are 28.3 for NS, 30.3 for rNS-gCnull, and 16.9 for NS-gCnull. NS and rNS-gCnull are significantly different from NS-gCnull (P < 0.01). (B) Vaginitis scores for the three viruses. NS-gCnull is significantly different from rNS-gCnull (P < 0.01) and NS (P = 0.02). Error bars represent ± SEM.
FIG. 4
The interaction between gC and complement is important for HSV-1 virulence in guinea pigs. C3D or complement-intact guinea pigs were inoculated with 5 × 103 PFU of NS-gCnull (A), rNS-gCnull (B), or NS (C). Vaginal swabs were taken at the indicated times, and titers were measured. AUC of NS-gCnull in complement-intact animals (3.6) is significantly different from that of C3D animals (12.7) (P = 0.02). AUCs of rescued or wild-type virus are not significantly different in complement-intact and C3D guinea pigs. Error bars represent ± SEM.
FIG. 5
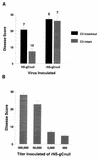
The interaction between gC and C3 is important for HSV-1 virulence in mice. (A) C57BL/6 C3 knockout or C57BL/6 parental strain complement-intact mice were inoculated intradermally with 5 × 105 PFU of NS-gCnull or rNS-gCnull virus. Disease scores were determined at the inoculation site by counting the number of lesions. Disease scores for NS-gCnull were 20.6 in C3 knockout mice and 7.4 in complement-intact mice (P < 0.0001). Disease scores for rNS-gCnull were 27.1 in C3 knockout mice and 26.1 in complement-intact mice (P = nonsignificant). The number of animals studied is indicated above each bar. (B) Dose-response studies of rNS-gCnull in C57BL/6 complement-intact mice inoculated with 5 × 105, 5 × 104, 5 × 103, or 5 × 102 PFU. Disease scores plotted represent the mean ± SEM of six mice per group (five mice in the 5 × 105 PFU group). The disease score with 5 × 103 PFU of rNS-gCnull was 6.9, which is comparable to the score of 7.4 shown in panel A for animals inoculated with 5 × 105 PFU of NS-gCnull virus. Error bars represent ± SEM.
Similar articles
- In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC.
Lubinski J, Wang L, Mastellos D, Sahu A, Lambris JD, Friedman HM. Lubinski J, et al. J Exp Med. 1999 Dec 6;190(11):1637-46. doi: 10.1084/jem.190.11.1637. J Exp Med. 1999. PMID: 10587354 Free PMC article. - Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine.
Awasthi S, Lubinski JM, Friedman HM. Awasthi S, et al. Vaccine. 2009 Nov 16;27(49):6845-53. doi: 10.1016/j.vaccine.2009.09.017. Epub 2009 Sep 15. Vaccine. 2009. PMID: 19761834 Free PMC article. - Herpes simplex virus glycoprotein C: molecular mimicry of complement regulatory proteins by a viral protein.
Huemer HP, Wang Y, Garred P, Koistinen V, Oppermann S. Huemer HP, et al. Immunology. 1993 Aug;79(4):639-47. Immunology. 1993. PMID: 8406590 Free PMC article. - Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone.
Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. Awasthi S, et al. J Virol. 2011 Oct;85(20):10472-86. doi: 10.1128/JVI.00849-11. Epub 2011 Aug 3. J Virol. 2011. PMID: 21813597 Free PMC article. - Immune responses to herpes simplex virus in guinea pigs (footpad model) and mice immunized with vaccinia virus recombinants containing herpes simplex virus glycoprotein D.
Aurelian L, Smith CC, Wachsman M, Paoletti E. Aurelian L, et al. Rev Infect Dis. 1991 Nov-Dec;13 Suppl 11:S924-34. doi: 10.1093/clind/13.supplement_11.s924. Rev Infect Dis. 1991. PMID: 1664130 Review.
Cited by
- Complement System Part II: Role in Immunity.
Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Merle NS, et al. Front Immunol. 2015 May 26;6:257. doi: 10.3389/fimmu.2015.00257. eCollection 2015. Front Immunol. 2015. PMID: 26074922 Free PMC article. Review. - Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant.
Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, Chiocca EA. Ikeda K, et al. J Virol. 2000 May;74(10):4765-75. doi: 10.1128/jvi.74.10.4765-4775.2000. J Virol. 2000. PMID: 10775615 Free PMC article. - Neuraminidase-mediated, NKp46-dependent immune-evasion mechanism of influenza viruses.
Bar-On Y, Glasner A, Meningher T, Achdout H, Gur C, Lankry D, Vitenshtein A, Meyers AFA, Mandelboim M, Mandelboim O. Bar-On Y, et al. Cell Rep. 2013 Apr 25;3(4):1044-50. doi: 10.1016/j.celrep.2013.03.034. Epub 2013 Apr 18. Cell Rep. 2013. PMID: 23602571 Free PMC article. - The relevance of complement to virus biology.
Blue CE, Spiller OB, Blackbourn DJ. Blue CE, et al. Virology. 2004 Feb 20;319(2):176-84. doi: 10.1016/j.virol.2003.11.029. Virology. 2004. PMID: 15015499 Free PMC article. Review. No abstract available. - Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease.
Saldanha CE, Lubinski J, Martin C, Nagashunmugam T, Wang L, van Der Keyl H, Tal-Singer R, Friedman HM. Saldanha CE, et al. J Virol. 2000 Aug;74(15):6712-9. doi: 10.1128/jvi.74.15.6712-6719.2000. J Virol. 2000. PMID: 10888608 Free PMC article.
References
- Burger R, Gordon J, Stevenson G, Ramadori G, Zanker B, Hadding U, Bitter-Suermann D. An inherited deficiency of the third component of complement, C3, in guinea pigs. Eur J Immunol. 1986;16:7–11. - PubMed
- Chrisp C E, Sunstrum J C, Averill D R, Jr, Levine M, Glorioso J C. Characterization of encephalitis in adult mice induced by intracerebral inoculation of herpes simplex virus type 1 (KOS) and comparison with mutants showing decreased virulence. Lab Investig. 1989;60:822–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous