T-cell apoptosis in inflammatory brain lesions: destruction of T cells does not depend on antigen recognition - PubMed (original) (raw)
T-cell apoptosis in inflammatory brain lesions: destruction of T cells does not depend on antigen recognition
J Bauer et al. Am J Pathol. 1998 Sep.
Abstract
Elimination of inflammatory T cells by apoptosis appears to play an important role in the down-regulation of inflammation in the central nervous system. Here we report that apoptosis of T lymphocytes occurs to a similar extent in different models of autoimmune encephalomyelitis. Apoptosis is restricted to cells located in the neuroectodermal parenchyma, thereby leaving T cells present in the brain's connective tissue compartments unharmed. Death of T cells in the parenchyma does not depend on antigen presentation by resident microglial cells or astrocytes. Adoptive transfer experiments with T lymphocytes carrying a specific genetic marker revealed that in the central nervous system these cells are destroyed regardless of their antigen specificity or state of activation. Although many of both antigen-dependent and -independent mechanisms in the induction of T-cell apoptosis may act simultaneously, our results suggest that the nervous system harbors a specific, currently undefined, mechanism that effectively eliminates infiltrating T lymphocytes.
Figures
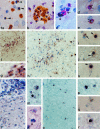
Figure 1.
a: Apoptosis in W3/13+ T cells. Apoptotic cells with a condensed nucleus (arrowhead) mostly are found singularly in the spinal cord parenchyma (MBP EAE, day 4; magnification, ×855). b: In some cases, apoptotic T cells are found in clusters of variable size (S100 EAE, day 4; ×855). c to e: Double staining for proliferation marker PCNA and W3/13. c shows a W3/13+ T cell (red, arrow) in close apposition to a PCNA+ nucleus (arrowhead) probably from a macrophage (MBP EAE, day 4; ×855). d and e: PCNA (black, arrowhead) and W3/13 (red) double-stained T cells. The homogenously stained nucleus with PCNA immunoreactivity against the nucleus (arrowheads) shows clear condensation, suggesting that these cells are undergoing apoptosis (MBP EAE, day 4; ×855). f: ISH for TK-tsA showing labeling of cells in the spinal cord of a TK-tsA transgenic rat with EAE (day 6) induced by TK-tsA MBP-specific T cells (×213). g: Higher magnification of f. All cells in the perivascular infiltrate show TK-tsA labeling in one or two distinct (brown) spots on the nucleus (×855). h to k: Transfer of TK-tsA MBP-specific T cells in normal Lewis rats. h: Low magnification of a Lewis rat with EAE induced by TK-tsA+ MBP-specific T cells on day 4 after transfer. High numbers of TK-tsA+ cells can be found around blood vessels and deeply infiltrated in the parenchyma (×336). i: Normal TK-tsA+ MBP cells are labeled with two clear distinct spots on the nucleus (arrow), whereas apoptotic cells (arrowhead) show a strong diffuse labeling over the entire nucleus (×855). j and k: ISH for TK-tsA showing two examples of apoptotic MBP-specific T cells in a Lewis rat EAE lesion that demonstrate the presence of apoptotic bodies (arrowheads) near the main part of the apoptotic cells (×855). l to n: ISH for TK-tsA. Transfer of Lewis MBP-specific T cells in TK-tsA rats (day 6). l: Low magnification of a longitudinal section of the spinal cord. High numbers of TK-tsA-labeled T cells are present in the meninges (m; ×266). m: TK-tsA-positive cells with the morphological appearance (diffuse staining over the entire nucleus) of apoptosis (arrowhead) can be found in between normal TK-tsA-labeled cells with one or two spots on the nucleus (×855). n: In addition to the TK-tsA-labeled apoptotic cells, TK-tsA-negative (MBP-specific) apoptotic cells (arrowhead) can also be found. In this case, the apoptotic cells lie in close apposition to a TK-tsA-labeled macrophage (×855). o and p: Transfer of activated TK-tsA OVA-specific T cells in a Lewis rat with EAE induced by Lewis MBP-specific T cells (day 4 after transfer). o: ISH for TK-tsA reveals the presence of many infiltrated OVA-specific T cells in the spinal cord (×266). p: ISH for TK-tsA in a higher magnification shows that besides normal TK-tsA+ OVA-specific T cells (two dots on the nucleus), apoptotic OVA-specific T cells can also be found (×855). q and r: Transfer of resting TK-tsA OVA-specific T cells in animals with EAE results in migration of low numbers of OVA-specific T cells in the spinal cord parenchyma (day 4 after transfer). Both normal OVA-specific T cells (q; ×855) and OVA-specific T cells with an apoptotic appearance (r; ×855) can be found.
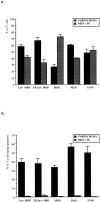
Figure 2.
Infiltration and apoptosis of T cells in various models of EAE. a: percentage of T cells (±SEM) in the parenchyma and in meninges/perivascular space in models of EAE induced by antigen-specific T-cell lines against MBP (cell line C1; Lew MBP), MOG, MAG, and S100. In addition, EAE was induced by TK-tsA MBP-specific T cells in Lewis rats (TK-tsA MBP). b: percentages (±SEM) of apoptotic T cells in the parenchyma and in the meninges/perivascular space in these different models of EAE.
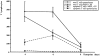
Figure 3.
Numbers of (apoptotic) T lymphocytes in parenchyma and meninges/perivascular space during the course of EAE. The average number (with SEM) of total T cells and apoptotic T cells was quantified in lumbar spinal cord cross sections on days 4, 6 and 9 of animals with MBP-T cell-induced EAE.
Comment in
- The fate of T cells in the brain: veni, vidi, vici and veni, mori.
Lehmann PV. Lehmann PV. Am J Pathol. 1998 Sep;153(3):677-80. doi: 10.1016/S0002-9440(10)65609-X. Am J Pathol. 1998. PMID: 9736016 Free PMC article. Review. No abstract available.
Similar articles
- Interaction of T lymphocytes with cerebral endothelial cells in vitro.
Wekerle H, Engelhardt B, Risau W, Meyermann R. Wekerle H, et al. Brain Pathol. 1991 Jan;1(2):107-14. doi: 10.1111/j.1750-3639.1991.tb00647.x. Brain Pathol. 1991. PMID: 1727014 Review. - Immune (dys)regulation in multiple sclerosis: role of the CD95-CD95 ligand system.
Zipp F, Krammer PH, Weller M. Zipp F, et al. Immunol Today. 1999 Dec;20(12):550-4. doi: 10.1016/s0167-5699(99)01545-5. Immunol Today. 1999. PMID: 10562705 Review. No abstract available.
Cited by
- CD24 controls expansion and persistence of autoreactive T cells in the central nervous system during experimental autoimmune encephalomyelitis.
Bai XF, Li O, Zhou Q, Zhang H, Joshi PS, Zheng X, Liu Y, Wang Y, Zheng P, Liu Y. Bai XF, et al. J Exp Med. 2004 Aug 16;200(4):447-58. doi: 10.1084/jem.20040131. J Exp Med. 2004. PMID: 15314074 Free PMC article. - Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions.
Schlitt BP, Felrice M, Jelachich ML, Lipton HL. Schlitt BP, et al. J Virol. 2003 Apr;77(7):4383-8. doi: 10.1128/jvi.77.7.4383-4388.2003. J Virol. 2003. PMID: 12634394 Free PMC article. - Co-localization of multiple antigens and specific DNA. A novel method using methyl methacrylate-embedded semithin serial sections and catalyzed reporter deposition.
Mueller M, Wacker K, Hickey WF, Ringelstein EB, Kiefer R. Mueller M, et al. Am J Pathol. 2000 Dec;157(6):1829-38. doi: 10.1016/S0002-9440(10)64822-5. Am J Pathol. 2000. PMID: 11106556 Free PMC article. - Blockade of sustained tumor necrosis factor in a transgenic model of progressive autoimmune encephalomyelitis limits oligodendrocyte apoptosis and promotes oligodendrocyte maturation.
Valentin-Torres A, Savarin C, Barnett J, Bergmann CC. Valentin-Torres A, et al. J Neuroinflammation. 2018 Apr 24;15(1):121. doi: 10.1186/s12974-018-1164-y. J Neuroinflammation. 2018. PMID: 29690885 Free PMC article. - Early treatment with anti-VLA-4 mAb can prevent the infiltration and/or development of pathogenic CD11b+CD4+ T cells in the CNS during progressive EAE.
Mindur JE, Ito N, Dhib-Jalbut S, Ito K. Mindur JE, et al. PLoS One. 2014 Jun 4;9(6):e99068. doi: 10.1371/journal.pone.0099068. eCollection 2014. PLoS One. 2014. PMID: 24896098 Free PMC article.
References
- Wekerle H, Linington C, Lassmann H, Meyermann R: Cellular immune reactivity within the CNS. Trends Neurosci 1986, 9:271-277
- Hickey H, Hsu BL, Kimura H: T-cell entry into the rat central nervous system. J Neurosci Res 1991, 28:254-260 - PubMed
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 - PubMed
- Stone SH: Transfer of allergic encephalomyelitis by lymph node cells in inbred guinea pigs. Science 1961, 134:619-620 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources