Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease - PubMed (original) (raw)
Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease
R O Weller et al. Am J Pathol. 1998 Sep.
Abstract
Cerebral amyloid angiopathy in Alzheimer's disease is characterized by deposition of amyloid beta (Abeta) in cortical and leptomeningeal vessel walls. Although it has been suggested that Abeta is derived from vascular smooth muscle, deposition of Abeta is not seen in larger cerebral vessel walls nor in extracranial vessels. In the present study, we examine evidence for the hypothesis that Abeta is deposited in periarterial interstitial fluid drainage pathways of the brain in Alzheimer's disease and that this contributes significantly to cerebral amyloid angiopathy. There is firm evidence in animals for drainage of interstitial fluid from the brain to cervical lymph nodes along periarterial spaces; similar periarterial channels exist in humans. Biochemical study of 6 brains without Alzheimer's disease revealed a pool of soluble Abeta in the cortex. Histology and immunocytochemistry of 17 brains with Alzheimer's disease showed that Abeta accumulates five times more frequently around arteries than around veins, with selective involvement of smaller arteries. Initial deposits of Abeta occur at the periphery of arteries at the site of the putative interstitial fluid drainage pathways. These observations support the hypothesis that Abeta is deposited in periarterial interstitial fluid drainage pathways of the brain and contributes significantly to cerebral amyloid angiopathy in Alzheimer's disease.
Figures
Figure 1.
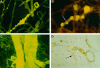
CAA of cortical vessels in AD. Aβ deposits associated with intracortical vessels, isolated by 10% sodium dodecyl sulfate treatment, stained by thioflavin S, and viewed in a fluorescence microscope (A to C). A: Globular deposits of amyloid are arranged along a small intracortical blood vessel. B: Linear deposits of amyloid (arrow) outline the wall of a small intracortical blood vessel between the globular deposits. C: Intracortical arteries showing transverse bands of amyloid deposition in the vessel walls. D: Paraffin section stained by immunocytochemistry for Aβ. Two leptomeningeal arteries can be seen on the right of the picture. In the larger vessel (top right), Aβ is deposited in the adventitial region and the media is intact. The smaller vessel sends a branch into the cortex; smooth muscle cells in the media of this artery are surrounded by amyloid deposits producing a band-like appearance similar to that seen in thioflavin S preparations. Amyloid is also present at the glia limitans on the surface of the cortex (arrow).
Figure 2.
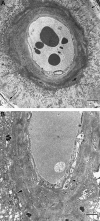
CAA of cortical vessels in AD. Transmission electron micrographs of intracortical arteries showing the pattern of deposition of amyloid in perivascular spaces and in the media. A: The perivascular space of this small intracortical artery is expanded by darkly stained wisps of amyloid, separating the glia limitans (arrows, top right, and bottom left) from the smooth muscle cells of the intact arterial media. Bar = 5 μm. B: Artery in which amyloid has been deposited within the perivascular space and between smooth muscle cells in the media. Glia limitans (arrowheads). Deposition of amyloid is heavier in the outer aspect of the media than in the inner aspects, where the smooth muscle cells remain better preserved (arrow). Bar = 5 μm.
Figure 3.
CAA of leptomeningeal arteries in AD. A: Isolated leptomeningeal artery showing heavy deposition of amyloid as transverse bands within its walls, associated with aneurysmal dilatation. Confocal microscopy: three-dimensional reconstruction, thioflavin S stain. B: Isolated leptomeningeal vessels viewed by phase-contrast microscopy showing a fusiform microaneurysm (left arrow) and a saccular microaneurysm (right arrow). C to F: Patterns of amyloid deposition in paraffin sections of leptomeningeal vessels and arachnoid stained with thioflavin S. C: Some smaller arteries show amyloid deposition throughout the thickness of their walls (left arrow), whereas other small vessels show complete absence of amyloid (right arrow). In the larger vessel, amyloid is deposited as a linear streak (middle arrow) in the adventitia and perivascular space. D: Leptomeningeal artery with a small streak of amyloid deposited at the junction of the media and adventitia (right arrow). The associated arachnoid mater also contains small streaks of amyloid (left arrow). E: Part of the wall of a leptomeningeal artery showing a linear circumferential deposit of amyloid at the junction of the adventitia and the media (arrow). F: Linear deposit of amyloid (arrow) in the wall of a leptomeningeal artery; the deposit is mostly in the adventitia near the perivascular space but also extends into the outer media.
Figure 4.
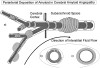
Diagram summarizing the pattern of the distribution of amyloid in CAA associated with AD. a: Aβ accumulates as globules or linear deposits in the perivascular spaces of small intracortical blood vessels or as transverse bands in the walls of larger intracortical arteries and in smaller leptomeningeal arteries. The severity of amyloid angiopathy decreases with increasing size of the artery, suggesting that Aβ is precipitated to a greater extent in the initial portions of the pathways draining ISF from the brain. b: With increasing deposition, Aβ surrounds smooth muscle cells in the media. c: Eventually, smooth muscle cells are lost and aneurysms may form.
Similar articles
- Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy.
Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Hawkes CA, et al. Brain Pathol. 2014 Jul;24(4):396-403. doi: 10.1111/bpa.12159. Brain Pathol. 2014. PMID: 24946077 Free PMC article. - Cerebral amyloid angiopathy: accumulation of A beta in interstitial fluid drainage pathways in Alzheimer's disease.
Weller RO, Massey A, Kuo YM, Roher AE. Weller RO, et al. Ann N Y Acad Sci. 2000 Apr;903:110-7. doi: 10.1111/j.1749-6632.2000.tb06356.x. Ann N Y Acad Sci. 2000. PMID: 10818495 Review. - Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease.
Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, Luehrs DC, Childress JL, Beach TG, Weller RO, Kokjohn TA. Roher AE, et al. Mol Med. 2003 Mar-Apr;9(3-4):112-22. Mol Med. 2003. PMID: 12865947 Free PMC article. - Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: defining the perivascular route for the elimination of amyloid beta from the human brain.
Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Preston SD, et al. Neuropathol Appl Neurobiol. 2003 Apr;29(2):106-17. doi: 10.1046/j.1365-2990.2003.00424.x. Neuropathol Appl Neurobiol. 2003. PMID: 12662319 - Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease.
Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Weller RO, et al. Brain Pathol. 2008 Apr;18(2):253-66. doi: 10.1111/j.1750-3639.2008.00133.x. Brain Pathol. 2008. PMID: 18363936 Free PMC article. Review.
Cited by
- Local perfusion of capillaries reveals disrupted beta-amyloid homeostasis at the blood-brain barrier in Tg2576 murine Alzheimer's model.
Hanafy AS, Lamprecht A, Dietrich D. Hanafy AS, et al. Fluids Barriers CNS. 2023 Nov 22;20(1):85. doi: 10.1186/s12987-023-00492-7. Fluids Barriers CNS. 2023. PMID: 37993886 Free PMC article. - Abeta aggregation and possible implications in Alzheimer's disease pathogenesis.
Bharadwaj PR, Dubey AK, Masters CL, Martins RN, Macreadie IG. Bharadwaj PR, et al. J Cell Mol Med. 2009 Mar;13(3):412-21. doi: 10.1111/j.1582-4934.2009.00609.x. J Cell Mol Med. 2009. PMID: 19374683 Free PMC article. Review. - Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy.
Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Hawkes CA, et al. Brain Pathol. 2014 Jul;24(4):396-403. doi: 10.1111/bpa.12159. Brain Pathol. 2014. PMID: 24946077 Free PMC article. - Exogenous Aβ seeds induce Aβ depositions in the blood vessels rather than the brain parenchyma, independently of Aβ strain-specific information.
Hamaguchi T, Kim JH, Hasegawa A, Goto R, Sakai K, Ono K, Itoh Y, Yamada M. Hamaguchi T, et al. Acta Neuropathol Commun. 2021 Sep 10;9(1):151. doi: 10.1186/s40478-021-01252-0. Acta Neuropathol Commun. 2021. PMID: 34507620 Free PMC article. - The role of cholesterol metabolism in Alzheimer's disease.
Sun JH, Yu JT, Tan L. Sun JH, et al. Mol Neurobiol. 2015;51(3):947-65. doi: 10.1007/s12035-014-8749-y. Epub 2014 May 18. Mol Neurobiol. 2015. PMID: 24838626 Review.
References
- Wisniewski HM, Wegiel J, Kotula L: Some neuropathological aspects of Alzheimer disease and its relevance to other disciplines. Neuropathol Appl Neurobiol 1996, 22:3-11 - PubMed
- Vinters HV, Wang ZZ, Secor DL: Brain parenchymal and microvascular amyloid in Alzheimer’s disease. Brain Pathol 1996, 6:179-195 - PubMed
- Shinkai Y: Amyloid β-proteins 1-40, and 1-42(43) in the soluble fraction of extra-, and intracranial blood vessels. Ann Neurol 1995, 38:421-428 - PubMed
- Cserr HF, Harling-Berg CJ, Knopf PM: Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol 1992, 2:269-276 - PubMed
- Boulton M, Young A, Hay J, Armstrong D, Flessner M, Schwartz M, Johnston M: Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol 1996, 22:325-333 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical