Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy - PubMed (original) (raw)
Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy
K Akassoglou et al. Am J Pathol. 1998 Sep.
Abstract
The scientific dogma that multiple sclerosis (MS) is a disease caused by a single pathogenic mechanism has been challenged recently by the heterogeneity observed in MS lesions and the realization that not all patterns of demyelination can be modeled by autoimmune-triggered mechanisms. To evaluate the contribution of local tumor necrosis factor (TNF) ligand/receptor signaling pathways to MS immunopathogenesis we have analyzed disease pathology in central nervous system-expressing TNF transgenic mice, with or without p55 or p75TNF receptors, using combined in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling and cell identification techniques. We demonstrate that local production of TNF by central nervous system glia potently and selectively induces oligodendrocyte apoptosis and myelin vacuolation in the context of an intact blood-brain barrier and absence of immune cell infiltration into the central nervous system parenchyma. Interestingly, primary demyelination then develops in a classical manner in the presence of large numbers of recruited phagocytic macrophages, possibly the result of concomitant pro-inflammatory effects of TNF in the central nervous system, and lesions progress into acute or chronic MS-type plaques with axonal damage, focal blood-brain barrier disruption, and considerable oligodendrocyte loss. Both the cytotoxic and inflammatory effects of TNF were abrogated in mice genetically deficient for the p55TNF receptor demonstrating a dominant role for p55TNF receptor-signaling pathways in TNF-mediated pathology. These results demonstrate that aberrant local TNF/p55TNF receptor signaling in the central nervous system can have a potentially major role in the aetiopathogenesis of MS demyelination, particularly in MS subtypes in which oligodendrocyte death is a primary pathological feature, and provide new models for studying the basic mechanisms underlying oligodendrocyte and myelin loss.
Figures
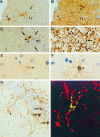
Figure 1.
An S100/CNPaselow/MAP-2low CNS glial precursor cell is the cellular source of murine TNF transgene expression in Tg6074 mice. Immunostaining of serial brain sections for TNF (A, arrowheads) and S100 (B, arrowheads) revealed that the Tg6074 transgene is expressed by a subpopulation of S100-positive cells. Immunostaining of serial brain sections for TNF (C) and MAP-2 (D), revealed that strongly MAP-2 positive neurons (D, arrows) are negative for TNF (C, arrows), while the TNF-positive cell (C, arrowhead) expressed lower levels of MAP-2 (D, arrowhead) when compared to the surrounding neurons (D, arrows). Immunostaining of serial brain sections for TNF (E) and CNPase (F) revealed that the TNF-positive cell also stained weakly for CNPase. (G) CNPase immunostaining of Tg6074 hippocampus demonstrated the weak reactivity of these highly branched precursor cells (arrowhead) when compared to the strong staining of adult oligodendrocytes (arrows). (H) Double immunofluorescence for CNPase (red) and TNF (green) examined by confocal microscopy demonstrated the co-localization (yellow) of the murine TNF transgene in CNPase positive cells. Magnifications: A, B ×792; C-F ×990; G ×335; H ×1608.
Figure 2.
Primary demyelination associated with oligodendrocyte apoptosis leads to chronic plaque formation in Tg6074 transgenic mice. Early primary demyelinating lesions develop by 4 weeks of age and are characterized by myelin vacuolation, as shown by LFB/PAS staining (A, arrow), and in situ hybridization for PLP mRNA (B, black) and immunocytochemistry for PLP protein (B, red); arrowheads show myelin vacuoles. Oligodendrocyte apoptosis revealed by fragmented nucleus as shown by MOG/hematoxylin at 2 weeks of age (C) and by a pyknotic nucleus as shown by CNPase/hematoxylin at 8 weeks of age (D). Confluent demyelinating plaques develop by 10 weeks of age (E-I). LFB/PAS staining of the cerebellum (E) revealed loss of myelin and the presence of numerous PAS positive macrophages which at the lesion edge contained myelin degradation products (E, asterisk; F). In situ hybridization for PLP mRNA (G, black) counterstained with anti-PLP (G, red) showed the loss of oligodendrocytes and myelin with a few remaining oligodendrocytes in the lesion area containing PLP mRNA (G, arrowhead). The borders of the cerebellar dentate nucleus (ND) are indicated. Bielschowsky silver staining revealed moderate axonal damage (H, asterisk) . CD3 immunostaining revealed T lymphocyte infiltration in the cerebellum (I). Magnifications: A, ×68; B, ×388; C, ×990; D, ×1232; E, ×68; F, ×266, G, ×99; H, ×308; I, ×161.
Figure 3.
Primary demyelination develops in Tg6074 transgenic mice. At 4 weeks of age, semithin sections revealed myelin vacuolation (A, arrows). Electron microscopy of the same area as Figure 3A ▶ reveals large vacuoles in the myelin sheath (B). At 9 weeks of age, semithin sections revealed active primary demyelination characterized by high numbers of phagocytic macrophages (C, arrowheads), and the presence of naked axons (C, open arrows). (D) Electron microscopy of the same area as Figure 3C ▶ shows a cluster of demyelinated axons in the close vicinity of phagocytic macrophages (M). In the middle a single myelinated axon can still be seen. Magnifications: A, ×740; B, ×4133; C, ×925; D, ×6125.
Figure 4.
Acute lesions with primary demyelination and axonal damage develop in TgK21 transgenic mice. Demyelination was observed in the spinal cord of TgK21 mice by 4 weeks of age, together with massive meningeal and parenchymal inflammation as shown by immunocytochemistry for PLP protein counterstained with hematoxylin (A). The outer border of the CNS parenchyma is indicated by a dotted line. Axonal loss was observed by Bielschowsky silver staining (B). GFAP immunostaining revealed astrocytic loss in the acute plaque (C) (arrowheads outline plaque). In the lesion several phagocytic macrophages were detected (D and E, arrowheads) that contain PLP positive degradation products (D, arrowhead). Toluidine blue-stained semithin resin sections of the spinal cord revealed that primary demyelination was specific for the CNS myelin leaving PNS myelin completely unaffected (E, arrows). Severe BBB disruption was shown by immunostaining with anti-Ig (F). Arrowheads indicate Ig deposition in the acute plaque. CD3 immunostaining revealed T cell infiltration in the lesions (G). CNPase/hematoxylin shows a CNPase positive oligodendrocyte with a pyknotic nucleus characteristic of apoptotic cell death (H). CNPase immunostaining counterstained with hematoxylin revealed rows of oligodendrocytes with pyknotic and fragmented nuclei characteristic of apoptosis (I, arrowheads). TUNEL (J, black) counterstained with anti-CNPase (J, red) confirmed that such oligodendrocytes were undergoing apoptosis. Magnifications: A and B ×99; C and F ×388; D and J, ×990; E, ×925; G, ×484; H, ×1895; I, ×1232.
Figure 5.
TNF-triggered CNS demyelination is dependent on the presence of the p55TNF receptor. Ten-week-old Tg6074p55−/− mice show normal myelin with a normal distribution of PLP protein (A). In contrast, the cerebellum from a 4-week-old Tg6074p75−/− mouse immunostained for PLP showed myelin vacuolation (B, arrowheads) and inflammation (B, asterisk) similar to that observed in normal Tg6074 mice of the same age. Staining for the lectin GSI-B4 in Tg6074p75−/− mice revealed microglial activation in the cerebellar white matter (C, arrowheads). CNPase immunostaining in Tg6074p75−/− mice revealed early nuclear changes characteristic of apoptosis (D) and a CNPase-positive oligodendrocyte with a pyknotic nucleus (E). Magnifications: A, ×161; B, ×388; C, ×792; D, ×1232; E, ×990.
Similar articles
- Tumor necrosis factor-alpha and its receptors contribute to apoptosis of oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy) sustaining chronic mechanical compression.
Inukai T, Uchida K, Nakajima H, Yayama T, Kobayashi S, Mwaka ES, Guerrero AR, Baba H. Inukai T, et al. Spine (Phila Pa 1976). 2009 Dec 15;34(26):2848-57. doi: 10.1097/BRS.0b013e3181b0d078. Spine (Phila Pa 1976). 2009. PMID: 19949368 - TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination.
Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. Arnett HA, et al. Nat Neurosci. 2001 Nov;4(11):1116-22. doi: 10.1038/nn738. Nat Neurosci. 2001. PMID: 11600888 - [Some aspects of histopathology in multiple sclerosis].
Kuhlmann T, Brück W. Kuhlmann T, et al. Rev Neurol. 2000 Jun 16-30;30(12):1208-12. Rev Neurol. 2000. PMID: 10935252 Review. Spanish. - Oligodendrocytes and the early multiple sclerosis lesion.
Prineas JW, Parratt JD. Prineas JW, et al. Ann Neurol. 2012 Jul;72(1):18-31. doi: 10.1002/ana.23634. Ann Neurol. 2012. PMID: 22829266 Review.
Cited by
- Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS.
Merson TD, Binder MD, Kilpatrick TJ. Merson TD, et al. Neuromolecular Med. 2010 Jun;12(2):99-132. doi: 10.1007/s12017-010-8112-z. Epub 2010 Mar 30. Neuromolecular Med. 2010. PMID: 20411441 Review. - Comparative gene expression analysis in mouse models for multiple sclerosis, Alzheimer's disease and stroke for identifying commonly regulated and disease-specific gene changes.
Tseveleki V, Rubio R, Vamvakas SS, White J, Taoufik E, Petit E, Quackenbush J, Probert L. Tseveleki V, et al. Genomics. 2010 Aug;96(2):82-91. doi: 10.1016/j.ygeno.2010.04.004. Epub 2010 May 7. Genomics. 2010. PMID: 20435134 Free PMC article. - Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped?
Pukos N, Goodus MT, Sahinkaya FR, McTigue DM. Pukos N, et al. Glia. 2019 Nov;67(11):2178-2202. doi: 10.1002/glia.23702. Epub 2019 Aug 24. Glia. 2019. PMID: 31444938 Free PMC article. Review. - Detecting neurodegenerative pathology in multiple sclerosis before irreversible brain tissue loss sets in.
Van Schependom J, Guldolf K, D'hooghe MB, Nagels G, D'haeseleer M. Van Schependom J, et al. Transl Neurodegener. 2019 Dec 9;8:37. doi: 10.1186/s40035-019-0178-4. eCollection 2019. Transl Neurodegener. 2019. PMID: 31827784 Free PMC article. Review. - Demyelination initiated by oligodendrocyte apoptosis through enhancing endoplasmic reticulum-mitochondria interactions and Id2 expression after compressed spinal cord injury in rats.
Huang SQ, Tang CL, Sun SQ, Yang C, Xu J, Wang KJ, Lu WT, Huang J, Zhuo F, Qiu GP, Wu XY, Qi W. Huang SQ, et al. CNS Neurosci Ther. 2014 Jan;20(1):20-31. doi: 10.1111/cns.12155. Epub 2013 Aug 13. CNS Neurosci Ther. 2014. PMID: 23937638 Free PMC article.
References
- Martin R, McFarland HF, McFarlin DE: Immunological aspects of demyelinating diseases. Annu Rev Immunol 1992, 10:153-187 - PubMed
- Brosnan CF, Raine CS: Mechanisms of immune injury in multiple sclerosis. Brain Pathol 1996, 6:243-257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases