Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases - PubMed (original) (raw)
Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases
K J Luzzi et al. Am J Pathol. 1998 Sep.
Abstract
In cancer metastasis, only a small percentage of cells released from a primary tumor successfully form distant lesions, but it is uncertain at which steps in the process cells are lost. Our goal was to determine what proportions of B16F1 melanoma cells injected intraportally to target mouse liver 1) survive and extravasate, 2) form micrometastases (4 to 16 cells) by day 3, 3) develop into macroscopic tumors by day 13, and 4) remain as solitary dormant cells. Using in vivo videomicroscopy, a novel cell accounting assay, and immunohistochemical markers for proliferation (Ki-67) and apoptosis (TUNEL), we found that 1) 80% of injected cells survived in the liver microcirculation and extravasated by day 3, 2) only a small subset of extravasated cells began to grow, with 1 in 40 forming micrometastases by day 3, 3) only a small subset of micrometastases continued to grow, with 1 in 100 progressing to form macroscopic tumors by day 13 (in fact, most micrometastases disappeared), and 4) 36% of injected cells remained by day 13 as solitary cancer cells, most of which were dormant (proliferation, 2%; apoptosis, 3%; in contrast to cells within macroscopic tumors: proliferation, 91%; apoptosis/necrosis, 6%). Thus, in this model, metastatic inefficiency is principally determined by two distinct aspects of cell growth after extravasation: failure of solitary cells to initiate growth and failure of early micrometastases to continue growth into macroscopic tumors.
Figures
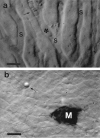
Figure 1.
Intravital videomicroscopic views of B16F1 melanoma cells in mouse liver. a: Intravascular melanoma cell (*) arrested due to size restriction within a sinusoid (S), shown 45 minutes after injection. Blood flow is blocked (→) in the region immediately downstream from the cell; sinusoids further downstream are being supplied by collateral flow. b: Micrometastasis (M) at the liver surface 3 days after cell injection, displaying melanin. A microsphere (→), slightly below the plane of focus, is also visible. Scale bars, 20 μm.
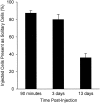
Figure 2.
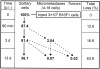
Survival of solitary melanoma cells in mouse liver after intraportal injection, assessed in thick tissue sections by a novel cell accounting technique. The loss of cells during the first 90 minutes was significant (P < 0.01), but the additional loss over the next 3 days was not (P = 0.21). By day 13, cell survival was significantly lower than at the other two time points (P < 0.01) but still amounted to more than one-third of the cells originally injected. Error bars represent SD.
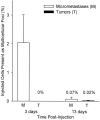
Figure 3.
Survival of injected melanoma cells as multicellular foci in liver, assessed in thick sections by the cell accounting technique. Only 2% of injected cells had formed micrometastases (4 to 16 cells) by day 3, and most of these had disappeared by day 13. Only 1 in 100 of the micrometastases, representing 0.02% of injected cells, had continued to grow into macroscopic tumors by day 13 (which virtually all form at the liver surface). Error bars represent SD.
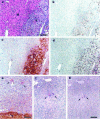
Figure 4.
Immunohistochemical staining of liver sections to assess apoptosis or proliferation of melanoma cells at 2 weeks after injection. a–d: Serial sections showing macroscopic tumor (T) and normal tissue (N). Bar, 100 μm. a: Hematoxylin and eosin. b: TUNEL assay shows that very few cells within the tumor were undergoing apoptosis. (The DNAse-positive controls, not shown, exhibited staining of virtually all cell nuclei.) c: S100 Ab identifies melanoma cells, which in this field of view were present only within the tumor. d: Ki-67 Ab shows that most cells within the tumor were proliferating. e: Examples of solitary melanoma cells (→) in normal tissue adjacent to a tumor (T), identified by S100 staining. These solitary cells did not stain with TUNEL or Ki-67 (data not shown), indicating that they were dormant. Bar, 50 μm. f and g: Examples of solitary melanoma cells (→) undergoing apoptosis. Serial sections; bar, 50 μm. Two cells identified by S100 (f) also stained positively with TUNEL (g), indicating apoptosis.
Figure 5.
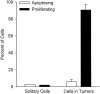
Percentages of melanoma cells undergoing apoptosis (TUNEL stain) or proliferation (Ki-67 stain) for solitary cells versus cells in tumors, at 2 weeks after injection. Data obtained by quantification from serial sections (see Figure 4 ▶ ) show dramatic differences in proliferation for solitary cells versus cells in tumors but very low levels of apoptosis in both instances. These results indicate that 95% of solitary cells were dormant versus only 3% in tumors.
Figure 6.
Flow chart summarizing survival data shows the multistep nature of metastatic inefficiency: percentages of injected cells remaining as solitary cells, or forming micrometastases or macroscopic tumors, at different times after injection (p.i.). (At 90 minutes, >85% of injected cells were intravascular, whereas by 3 days, >80% had completed extravasation.) Note the slow loss of solitary cells with time. Dotted arrows indicate possible origins of micrometastases and macroscopic tumors. Two distinct steps after extravasation were principal determinants of metastatic inefficiency: failure of solitary cells to initiate growth and failure of micrometastases to continue growth into macroscopic tumors.
References
- Weinstat-Saslow D, Steeg PS: Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB J 1994, 8:401-407 - PubMed
- Fidler IJ, Ellis LM: The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 1994, 79:185-188 - PubMed
- Liotta LA, Stetler-Stevenson WG: Principles of molecular cell biology of cancer: cancer metastasis. DeVita VT, Jr Hellman S Rosenberg SA eds. Cancer: Principles and Practice of Oncology. 1993, :pp 134-149 Lippincott, Philadelphia
- Weiss L: Metastatic inefficiency. Adv Cancer Res 1990, 54:159-211 - PubMed
- Fidler IJ: Metastasis: quantitative analysis of the distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 1970, 45:773-782 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases