Critical role of axonal A-type K+ channels and axonal geometry in the gating of action potential propagation along CA3 pyramidal cell axons: a simulation study - PubMed (original) (raw)
Critical role of axonal A-type K+ channels and axonal geometry in the gating of action potential propagation along CA3 pyramidal cell axons: a simulation study
I L Kopysova et al. J Neurosci. 1998.
Abstract
A model of CA3 pyramidal cell axons was used to study a new mode of gating of action potential (AP) propagation along the axon that depends on the activation of A-type K+ current (Debanne et al., 1997). The axonal membrane contained voltage-dependent Na+ channels, K+ channels, and A-type K+ channels. The density of axonal A-channels was first determined so that (1) at the resting membrane potential an AP elicited by a somatic depolarization was propagated into all axon collaterals and (2) propagation failures occurred when a brief somatic hyperpolarization preceded the AP induction. Both conditions were fulfilled only when A-channels were distributed in clusters but not when they were homogeneously distributed along the axon. Failure occurs in the proximal part of the axon. Conduction failure could be determined by a single cluster of A-channels, local decrease of axon diameter, or axonal elongation. We estimated the amplitude and temporal parameters of the hyperpolarization required for induction of a conduction block. Transient and small somatic hyperpolarizations, such as simulated GABAA inhibitory postsynaptic potentials, were able to block the AP propagation. It was shown that AP induction had to occur with a short delay (<30 msec) after the hyperpolarization. We discuss the possible conditions in which such local variations of the axon geometry and A-channel density may occur and the incidence of AP propagation failures on hippocampal network properties.
Figures
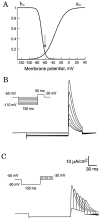
Fig. 1.
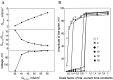
Properties of A-current in an isolated compartment of membrane. A, Activation and inactivation curves of A-current. Note that at the RMP (vertical arrow), most of the channels were inactivated. B, Level of the hyperpolarization and recovery from inactivation in simulations of voltage-clamp-mode experiments. The holding potential was −50 mV. A-current was activated after a 150 msec hyperpolarizing prepulse by the depolarizing pulse to +30 mV (50 msec duration). Hyperpolarizing voltage command ranged between −50 and −110 mV (inset). A-current increased significantly with the level of the hyperpolarization. C, Delay between the hyperpolarizing prepulse and depolarizing command. The membrane was hyperpolarized to −90 mV during the prepulse. The depolarizing command (+30 mV) was applied 0, 10, 20, 30, 40, and 50 msec after the end of the hyperpolarization (inset). The shorter the delay of delivery of the depolarization, the larger the peak of A-current.
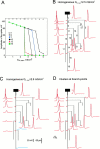
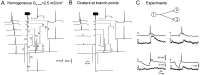
Fig. 2.
Propagation of the AP along the axon with different distributions of A-channels. A, Homogeneous distribution. Amplitudes of the AP in the area of the spike generation (branch 1) and in five axonal collaterals (2, 7, 10, 12, and 16; see_B_) as a function of the density of A-channels. At low density (_G_A,max ≤ 2.5 mS/cm2), the AP propagated into all axonal terminals. At _G_A,max = 2.6 mS/cm2 conduction failed in branch 16 of the principal axon and collateral 12 was obtained. With further increase of_G_A,max, more terminals were blocked, and at _G_A,max = 4.5 mS/cm2 the AP failed to reach any terminal.B, Details of AP propagation within the whole axonal arborization when A-channels were distributed homogeneously with a low density (_G_A,max = 2.5 mS/cm2). Action potentials were elicited by a brief current-induced depolarization (inflection on the rising phase of the AP). C, Propagation failed in the principal axon (16) and in the collateral 12 (blue traces) when A-channel density was increased to 2.6 mS/cm2.D, Successful propagation when hot spots of A-channels (_G_A,max = 4.1 mS/cm2) at the branch points were added to a low homogeneous density of A-channels (_G_A,max = 1.2 mS/cm2).
Fig. 3.
Propagation of the AP after a hyperpolarizing prepulse. A, Simulations for homogeneous distribution of A-channels (G_A,max = 2.5 mS/cm2). AP was elicited after a 20 msec hyperpolarizing prepulse of 9.5 mV. No conduction failure was induced.B, Clustered distribution as in Figure2_D. The same hyperpolarization induced propagation failures along the principal axon (branch 16) and collateral 12 but not in adjacent collaterals (2, 7, and 10).C, Selective conduction block in CA3 cells of hippocampal slice cultures. Pairs of two monosynaptically CA3 pyramidal cells were recorded intracellularly. Presynaptic AP induced in the cell (1) at the RMP evoked an EPSP in cell (2). When cell (1) was phasically hyperpolarized, no EPSP was elicited in the cell (2). The electrode was then removed from the cell (2) and inserted in the cell (3). Even in the presence of phasic hyperpolarization, the presynaptic AP always evoked an EPSP. AP thus failed in the axonal collateral of cell (1) to cell (2), but not in the branch to cell (3).
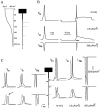
Fig. 4.
Location of the AP failure along the principal axon. A, Electrotonic attenuation along the principal axon of a steady-state hyperpolarization to −69.5 mV induced at the soma. Note that downstream from the third branch point (>200 μm), the membrane was almost not polarized. B, Time courses of the membrane potential in the soma (_V_m, top) and densities of A-current (_I_A,middle) and Na+ current (_I_Na, bottom) in response to a depolarization elicited either from the RMP (left) or after the hyperpolarizing prepulse (right). C, Time courses of the membrane potential (_V_m, left) and densities of the A-current (_I_A,middle) and Na+ current (_I_Na, right) were compared along the axon without (dashed lines) and with a hyperpolarizing prepulse (solid lines). All signals have been expanded over a duration of 5.5 msec (B, horizontal bar). Note the difference in the vertical scales for the Na+ currents in the soma (B), in the area of spike initiation, and in the rest of the axon.
Fig. 5.
Effect of removal of a single cluster on propagation failure. The control configuration was indicated by a_dashed line_. When the cluster of A-channel was removed from the second branch point (indicated by the dashed circle), the AP propagated successfully into all axon terminals (solid line).
Fig. 6.
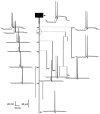
Effect of axon elongation and reduction of the axonal diameter on propagation failure. A, Control configuration. B, The length of axonal branch 9 was increased from 50 (a, b in A) to 100 μm (a, b’ in B). As a result, the AP propagated into all axonal collaterals. C, The diameter was decreased by 0.1 μm in three branches labeled by × (9, 10, and 11). Propagation into the whole axonal arborization was achieved.
Fig. 7.
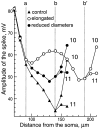
Comparison of AP amplitudes in three morphological configurations shown in Figure 6: control, with elongated branch and with decreased diameters. Part of the modeled structure included branches 3 and 9 and the first 20 μm of branches 10 and 11. Labeling of the branch points a, b, and_b’_ is the same as in Figure 6. In the control (filled triangles), the amplitude decreased in the vicinity of branch points a and b. The amplitude was sufficient to allow active propagation into branch 10 but not into branch 11. When the distance between the two branch points was increased (a, b’, empty circles), the amplitude of the AP was able to recover after passing the bifurcation_a_ (110 μm). Approaching b’, the AP amplitude decreased. Active propagation, however, was achieved in both branches after b’ (200 μm). For decreased diameters (filled circles), the amplitude drop was also weaker than in control, and active propagation into both collaterals (10 and 11) was observed.
Fig. 8.
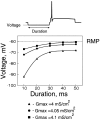
Amplitude and duration of the hyperpolarization required for the conduction failure. For different durations of the hyperpolarizing prepulse (10–50 msec), the minimal level of the hyperpolarization allowing a conduction failure was determined. Three densities of the A-channels in the hot spots were considered (_G_A,max = 4, 4.05, and 4.1 mS/cm2). The hyperpolarization required for deinactivation of the A-channels decreased when the duration increased. Note the saturation for durations larger than 40 msec. The required hyperpolarization increased significantly with a small decrease in the A-channel density. For a 10 msec prepulse, hyperpolarizations deeper than −67 mV were found to be sufficient to produce a conduction block when the density at the hot spots was 4.1 mS/cm2. For a smaller density of the A-channels (4.05 mS/cm2), hyperpolarization had to be at least −72 mV to provide a propagation failure. With further decrease in the A-channel density (4 mS/cm2), a conduction failure was obtained at −92 mV.
Fig. 9.
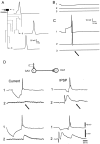
Critical delay between the hyperpolarization and AP induction (_G_A,max = 4.05 mS/cm2). A, Time courses of the transmembrane potential (_V_m,soma,top traces) and density of A-current (_V_A,soma, middle traces) in the soma, and potential in terminal 16 of the principal axon (_V_m,terminal, bottom traces) were calculated for different delays between the hyperpolarization and AP induction. Hyperpolarizing prepulse was 30 mV and its duration was 20 msec. A conduction block along the principal axon was observed when hyperpolarizing prepulse preceded the AP by 1 msec. B, Similar results were obtained for a delay of 25 msec. C, When the delay was increased to 35 msec, propagation into the principal axon was successful. D, When a 5 msec subthreshold depolarization was added to the configuration in B (25 msec delay), the AP propagated normally along the principal axon. E, Graph of the peak amplitude of A-current during the AP (shown in A–D) as a function of the delay. Note that for a delay of 25 msec, the peak amplitude was significantly reduced when a small depolarization event was added. F, Experimental results. Two monosynaptically coupled CA3 pyramidal cells were recorded intracellularly. When the hyperpolarizing prepulse preceded the AP by 15 msec in the cell (1), no EPSP was elicited in the cell (2). Propagation failure was not observed when the delay was increased to 35 msec.
Fig. 10.
Conduction block induced by a somatic IPSP (_G_A,max = 4.05 mS/cm2).A, GABAA-receptor synaptic input was placed on the soma. Without synaptic activation, the AP elicited at the soma (1) propagated into both collateral (2) and principal axon (3).B, Membrane potentials in the soma (1), collateral (2), and principal axon (3) in response to activation of the synaptic input. No hyperpolarization was detected in the collateral and principal axon. C, The AP failed to propagate into the principal axon (arrow on trace 3) when it was elicited at the maximum of the IPSP (see trace 1). It was still able to propagate into the collateral (2). D, Experimental conduction block. Two pyramidal cells were recorded intracellularly in areas CA3 (1) and CA1 (2). Similar effects were observed for the current-induced (right) and synaptically induced (left) hyperpolarizations of the cell (1). When the hyperpolarization preceded the presynaptic AP by <10 msec (_top_), no EPSP was observed postsynaptically (_arrows_). When the delay between the hyperpolarizing prepulse and the presynaptic AP was >30 msec (bottom), an EPSP was elicited for both types of hyperpolarization, indicating normal AP conduction into the presynaptic axon. The complex response before the unitary EPSP in the cell (2) resulted from activation of Schaffer collaterals.
Fig. 11.
Role of the density and kinetics of Na+ channels. A, Variation of the density. The density of Na+ channels (_G_Na,max) varied between 35 and 60 mS/cm2 in the soma and along the axon. In the area of spike generation the density of Na+ channels was 30 times higher. Top, The density of A-channels (_G_A,max) in the hot spots was calculated from the condition of successful spike propagation into all axonal collaterals at the RMP. _G_A,maxincreases with the increase of _G_Na,max.Middle, The ratio_G_Na,max/_G_A,maxwas calculated from the data presented in the top. To higher density of Na+ channels corresponded higher_G_A,max and lower_G_Na,max/_G_A,max.Bottom, The level of the hyperpolarization required to induce a failure of the spike propagation into the principal axon. The duration of the hyperpolarization was 20 msec. For_G_Na,max >40 mS/cm2, the required level of the hyperpolarization was found to be lower than −75 mV. It suggests that for this range of_G_Na,max, IPSPs or AHPs may induce conduction failures. B, Variation of the kinetics. For different kinetics of Na+ channels the AP amplitudes in five terminals were calculated. Simulations were performed in the absence of the hyperpolarizing prepulse. The time-constants of activation and inactivation of Na+ channels were multiplied by a scale factor presented on the abscissa. Scale factor equal to 1 corresponded to kinetics used in all previous simulations. For slower kinetics of Na+ channels (scale factors >1), amplitudes of the AP were larger, and spikes reached all terminals. For faster kinetics, the amplitude of the AP in the area of spike generation decreased, and the AP failed to reach some (scale factors ranging between 0.2 and 0.9) or all (scale factor of 0.1) terminals.
Similar articles
- Gating of action potential propagation by an axonal A-like potassium conductance in the hippocampus: a new type of non-synaptic plasticity.
Debanne D, Kopysova IL, Bras H, Ferrand N. Debanne D, et al. J Physiol Paris. 1999 Sep-Oct;93(4):285-96. doi: 10.1016/s0928-4257(00)80057-1. J Physiol Paris. 1999. PMID: 10574118 Review. - Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons.
Magee JC. Magee JC. J Neurosci. 1998 Oct 1;18(19):7613-24. doi: 10.1523/JNEUROSCI.18-19-07613.1998. J Neurosci. 1998. PMID: 9742133 Free PMC article. - Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus.
Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Debanne D, et al. Nature. 1997 Sep 18;389(6648):286-9. doi: 10.1038/38502. Nature. 1997. PMID: 9305843 - Action potential initiation and propagation in CA3 pyramidal axons.
Meeks JP, Mennerick S. Meeks JP, et al. J Neurophysiol. 2007 May;97(5):3460-72. doi: 10.1152/jn.01288.2006. Epub 2007 Feb 21. J Neurophysiol. 2007. PMID: 17314237 - Neuronal signaling in central nervous system.
Shu Y. Shu Y. Sheng Li Xue Bao. 2011 Feb 25;63(1):1-8. Sheng Li Xue Bao. 2011. PMID: 21340428 Review.
Cited by
- Branch specific and spike-order specific action potential invasion in basal, oblique, and apical dendrites of cortical pyramidal neurons.
Zhou WL, Short SM, Rich MT, Oikonomou KD, Singh MB, Sterjanaj EV, Antic SD. Zhou WL, et al. Neurophotonics. 2015 Apr;2(2):021006. doi: 10.1117/1.NPh.2.2.021006. Epub 2014 Dec 29. Neurophotonics. 2015. PMID: 26157997 Free PMC article. - Axonal properties determine somatic firing in a model of in vitro CA1 hippocampal sharp wave/ripples and persistent gamma oscillations.
Traub RD, Schmitz D, Maier N, Whittington MA, Draguhn A. Traub RD, et al. Eur J Neurosci. 2012 Sep;36(5):2650-60. doi: 10.1111/j.1460-9568.2012.08184.x. Epub 2012 Jun 15. Eur J Neurosci. 2012. PMID: 22697272 Free PMC article. - A presynaptic source drives differing levels of surround suppression in two mouse retinal ganglion cell types.
Swygart D, Yu WQ, Takeuchi S, Wong ROL, Schwartz GW. Swygart D, et al. Nat Commun. 2024 Jan 18;15(1):599. doi: 10.1038/s41467-024-44851-w. Nat Commun. 2024. PMID: 38238324 Free PMC article. - Quantitative morphometry of electrophysiologically identified CA3b interneurons reveals robust local geometry and distinct cell classes.
Ascoli GA, Brown KM, Calixto E, Card JP, Galván EJ, Perez-Rosello T, Barrionuevo G. Ascoli GA, et al. J Comp Neurol. 2009 Aug 20;515(6):677-95. doi: 10.1002/cne.22082. J Comp Neurol. 2009. PMID: 19496174 Free PMC article. - Axonal propagation: does the spike stop here?
Debanne D, Russier M. Debanne D, et al. J Physiol. 2003 May 1;548(Pt 3):663. doi: 10.1113/jphysiol.2002.037812. Epub 2003 Mar 14. J Physiol. 2003. PMID: 12640012 Free PMC article. No abstract available.
References
- Alonso G, Widmer H. Clustering of Kv4.2 potassium channels in supraoptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
- Andersen P, Silfvenius H, Sundberg SH, Sveen O, Wigström H. Functional characterization of unmyelinated fibres in the hippocampal cortex. Brain Res. 1978;144:11–18. - PubMed
- Bernander O, Koch C, Douglas RJ. Amplification and linearization of distal synaptic input to cortical pyramidal cells. J Neurophysiol. 1994;72:2743–2753. - PubMed
- Bielefeldt K, Jackson MB. A calcium-activated potassium channel causes frequency-dependent action potential failures in a mammalian nerve terminal. J Neurophysiol. 1993;70:284–298. - PubMed
- Bienenstock E. A model of neocortex. Network. 1995;6:179–224.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous