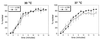Two yeast nuclear pore complex proteins involved in mRNA export form a cytoplasmically oriented subcomplex - PubMed (original) (raw)
Two yeast nuclear pore complex proteins involved in mRNA export form a cytoplasmically oriented subcomplex
M E Hurwitz et al. Proc Natl Acad Sci U S A. 1998.
Abstract
We sublocalized the yeast nucleoporin Nup82 to the cytoplasmic side of the nuclear pore complex (NPC) by immunoelectron microscopy. Moreover, by in vitro binding assays we showed that Nup82 interacts with the C-terminal region of Nup159, a yeast nucleoporin that previously was also localized to the cytoplasmic side of the NPC. Hence, the two nucleoporins, Nup82 and Nup159, form a cytoplasmically oriented subcomplex that is likely to be part of the fibers emanating from the cytoplasmic ring of the NPC. Overexpression of Rss1/Gle1, a putative nucleoporin and/or mRNA transport factor, was shown previously to partially rescue depletion of Nup159. We show here that overexpression of Rss1/Gle1 also partially rescued depletion of Nup82. Depletion of either Nup82, Nup159, or Rss1/Gle1 was shown previously to inhibit mRNA export. As was reported previously for depletion of Nup159 or of Rss1/Gle1, we show here that depletion of Nup82 has no detectable effect on classical nuclear localization sequence-mediated nuclear import. In summary, the nucleoporins Nup159 and Nup82 form a cytoplasmically oriented subcomplex of the NPC that is likely associated with Rss1/Gle1; this complex is essential for RNA export, but not for classical nuclear localization sequence-mediated nuclear protein import.
Figures
Figure 1
Depletion of Nup82 has no significant effect on nuclear protein import. NUP82-wt and NUP82-Δ108 yeast containing pGFP-URA were grown to mid-log phase and then incubated at 30°C or 37°C for 3 hr and tested in the NLS-GFP nuclear import assay.
Figure 2
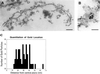
Immunoelectron microscopic localization of Nup82 to the cytoplasmic side of the NPC in isolated crude nuclear envelopes. (A) Side view. (Bar = 150 nm.) (B) Front view. (Bar = 55 nm.) (C) Quantitation of distance of gold particles from the NPC. The distance measured is a straight line perpendicular to the central plane of the NPC. Forty-eight particles were measured, and the mean and SD were found to be 29.9 nm and 10.9 nm, respectively.
Figure 3
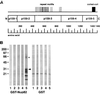
Nup82 binds to the coiled-coil domain of Nup159. (A) Nup159 was expressed in Escherichia coli in five different segments, p159-1, amino acid residues 1–175; p159-2, residues 176–440; p159-3, residues 441–876; p159-4, residues 877-1222; p159-5, residues 1223–1460. The position of the FXFG peptide repeats, coiled coil, and amino acid number are shown [modified from Kraemer et al. (17)]. (B) Fractions containing the expressed proteins were subjected to SDS/PAGE, transferred to nitrocellulose, incubated with GST-Nup82 or GST alone, and then incubated with a polyclonal rabbit anti-GST antibody. The arrowhead shows where p159-5 migrates. The asterisk is at approximately double the molecular weight of p159-5.
Figure 4
Overexpression of Rss1/Gle1 partially rescues the ts phenotype seen in NUP82-Δ108U yeast. The (+) rows contain NUP82-Δ108U yeast harboring pVDP7 (which overexpresses Rss1/Gle1). The (−) rows contain NUP82-Δ108U yeast harboring pRS315 (empty plasmid). Cells were grown in selective medium (-LEU) at 30°C overnight. Equivalent numbers of cells and serial 10-fold dilutions (based on OD600) were plated on either complete medium (YPD) or selective medium (-LEU) and incubated at 30°C or 37°C for 4 days.
Similar articles
- Functional characterization of a Nup159p-containing nuclear pore subcomplex.
Belgareh N, Snay-Hodge C, Pasteau F, Dagher S, Cole CN, Doye V. Belgareh N, et al. Mol Biol Cell. 1998 Dec;9(12):3475-92. doi: 10.1091/mbc.9.12.3475. Mol Biol Cell. 1998. PMID: 9843582 Free PMC article. - Structural and functional analysis of an essential nucleoporin heterotrimer on the cytoplasmic face of the nuclear pore complex.
Yoshida K, Seo HS, Debler EW, Blobel G, Hoelz A. Yoshida K, et al. Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16571-6. doi: 10.1073/pnas.1112846108. Epub 2011 Sep 19. Proc Natl Acad Sci U S A. 2011. PMID: 21930948 Free PMC article. - The nuclear pore complex.
Davis LI. Davis LI. Annu Rev Biochem. 1995;64:865-96. doi: 10.1146/annurev.bi.64.070195.004245. Annu Rev Biochem. 1995. PMID: 7574503 Review. - The nuclear pore complex.
Hurt EC. Hurt EC. FEBS Lett. 1993 Jun 28;325(1-2):76-80. doi: 10.1016/0014-5793(93)81417-x. FEBS Lett. 1993. PMID: 8513897 Review.
Cited by
- RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase.
Askjaer P, Bachi A, Wilm M, Bischoff FR, Weeks DL, Ogniewski V, Ohno M, Niehrs C, Kjems J, Mattaj IW, Fornerod M. Askjaer P, et al. Mol Cell Biol. 1999 Sep;19(9):6276-85. doi: 10.1128/MCB.19.9.6276. Mol Cell Biol. 1999. PMID: 10454574 Free PMC article. - The cytoplasmic filaments of the nuclear pore complex are dispensable for selective nuclear protein import.
Walther TC, Pickersgill HS, Cordes VC, Goldberg MW, Allen TD, Mattaj IW, Fornerod M. Walther TC, et al. J Cell Biol. 2002 Jul 8;158(1):63-77. doi: 10.1083/jcb.200202088. Epub 2002 Jul 8. J Cell Biol. 2002. PMID: 12105182 Free PMC article. - Sending the message: specialized RNA export mechanisms in trypanosomes.
Obado SO, Rout MP, Field MC. Obado SO, et al. Trends Parasitol. 2022 Oct;38(10):854-867. doi: 10.1016/j.pt.2022.07.008. Epub 2022 Aug 24. Trends Parasitol. 2022. PMID: 36028415 Free PMC article. Review. - Assembly and preferential localization of Nup116p on the cytoplasmic face of the nuclear pore complex by interaction with Nup82p.
Ho AK, Shen TX, Ryan KJ, Kiseleva E, Levy MA, Allen TD, Wente SR. Ho AK, et al. Mol Cell Biol. 2000 Aug;20(15):5736-48. doi: 10.1128/MCB.20.15.5736-5748.2000. Mol Cell Biol. 2000. PMID: 10891509 Free PMC article. - The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human.
Katahira J, Strässer K, Podtelejnikov A, Mann M, Jung JU, Hurt E. Katahira J, et al. EMBO J. 1999 May 4;18(9):2593-609. doi: 10.1093/emboj/18.9.2593. EMBO J. 1999. PMID: 10228171 Free PMC article.
References
- Yang Q, Rout M P, Akey C W. Mol Cell. 1998;1:223–234. - PubMed
- Rout M P, Wente S R. Trends Cell Biol. 1994;4:357–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials