Expression of the mitotic motor protein Eg5 in postmitotic neurons: implications for neuronal development - PubMed (original) (raw)
Expression of the mitotic motor protein Eg5 in postmitotic neurons: implications for neuronal development
L Ferhat et al. J Neurosci. 1998.
Abstract
It is well established that the microtubules of the mitotic spindle are organized by a variety of motor proteins, and it appears that the same motors or closely related variants organize microtubules in the postmitotic neuron. Specifically, cytoplasmic dynein and the kinesin-related motor known as CHO1/MKLP1 are used within the mitotic spindle, and recent studies suggest that they are also essential for the establishment of the axonal and dendritic microtubule arrays of the neuron. Other motors are required to tightly regulate microtubule behaviors in the mitotic spindle, and it is attractive to speculate that these motors might also help to regulate microtubule behaviors in the neuron. Here we show that a homolog of the mitotic kinesin-related motor known as Eg5 continues to be expressed in rodent neurons well after their terminal mitotic division. In neurons, Eg5 is directly associated with the microtubule array and is enriched within the distal regions of developing processes. This distal enrichment is transient, and typically lost after a process has been clearly defined as an axon or a dendrite. Strong expression can resume later in development, and if so, the protein concentrates within newly forming sprouts at the distal tips of dendrites. We suggest that Eg5 generates forces that help to regulate microtubule behaviors within the distal tips of developing axons and dendrites.
Figures
Fig. 1.
Cloning and characterization of Eg5 in mouse cells (MmEg5). A, Alignment of MmEg5 and HsEg5 protein sequences. Amino acids are shown using the single-letter code. The human sequence (Blangy et al., 1995) is shown only when it differs from the mouse sequence. Identities are indicated by dashes, and conservative substitutions (T/S, E/N/D/Q, K/R, Y/F/W, L/V/I/M) are shown by dots. The amino-terminal domain contains the consensus motifs that are normally found in motor domains of kinesin-related proteins, including YGQTXXGK(T/S), NXXSSRSH, and DLAGXE (boxes). Vertical bars mark the boundaries of the motor, link, stalk, and tail domains. Another group has recently published partial sequence information from the N-terminal region of MmEg5 that is almost identical to ours but contains a small number of nucleotide differences that result in six amino acid substitutions and one additional amino acid (Nakagawa et al., 1997). The asterisk indicates threonine (T), a site that can be phosphorylated presumably by cdc2 kinase. The consensus motifs of kinesin-like proteins are boxed, and the leucine zipper motif is underlined. B, Diagram showing the homologies (similar/identical amino acids) in different domains of MmEg5 and HsEg5. BESTFIT was used to find the best segments of similarities between the two sequences (Devereux et al., 1984).C, Coiled coil structure predicted by the algorithm ofLupas et al. (1991).
Fig. 2.
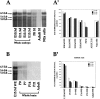
Expression of MmEg5 mRNAs in mouse tissues and cultured cells determined by Northern blot analyses. Total RNA (30 μg/lane) isolated from whole embryo at E10.5, E11.5, E13.5, E15.5 (A, lanes 1_–_4), from small intestine at P0 and adult (lanes 5,6), from whole brain at P0, P7, P14, P21, and adult (B, lanes 2_–_6), and from cultured mouse neuroblastoma cells (used as a positive control; A,lane 7) was electrophoresed in a formaldehyde 1% agarose gel, transferred to a nylon membrane, and then probed with radioactively labeled MmEg5 cDNA (see Materials and Methods). The transcripts (5.0, 5.6, and 6.5 kb) detected in whole embryo, small intestine, and whole brain were identical in size to those found in neuroblastoma cells. The histograms _A_′ and_B_′ show the changes in the levels of Eg5 mRNAs in different tissues during their development.
Fig. 3.
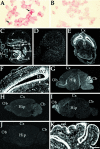
Expression of MmEg5 mRNAs in mouse tissues and cultured cells determined by in situ hybridization.A and B show cultured mouse neuroblastoma cells hybridized with the MmEg5 antisense and sense riboprobes, respectively. Autoradiographs C and D are representative of the hybridization pattern obtained with MmEg5 antisense and sense riboprobes, respectively, at E15.5. Hybridization signal was detected within the submandibular salivary gland (sg), cartilage primordium of the body of the hyoid bone (cb), thymus gland (tg), liver (l), gut (g), heart (h), tongue (t), kidney (k), lung (lu), and epithelial cells of the hindlimbs (hl). Shown in_E_ and F, respectively, are a film autoradiograph and corresponding dark-field illumination of a transverse section of the cerebral cortex (Cx) at E15.5 obtained with the MmEg5 antisense riboprobe. Et_indicates epithalamus, and SVZ indicates subventricular zone. LV indicates lateral ventricle. Autoradiographs_G_–_I are representative of the hybridization patterns obtained with MmEg5 antisense riboprobe at P7, P21, and adult mouse brain (Ad), respectively.Cb, cerebellum; Hip, hippocampus;Ob, olfactory bulb. Autoradiograph J is representative of the adult mouse brain hybridization pattern obtained with MmEg5 sense riboprobe. K, Dark-field illumination of the cerebellum hybridized for MmEg5 at P7. At P7, the external granular cell layer (egl) and the internal granular cell layer (igl) are labeled. Scale bar:A, B, 6 μm; C,D, 0.3 cm; E, 0.1 cm;F_–_K, 0.2 cm.
Fig. 4.
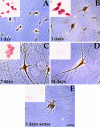
Expression of Eg5 mRNAs in cultured rat sympathetic neurons determined by in situ hybridization. Cultured sympathetic neurons were hybridized with either the radioactive (large panels) or with the digoxygenin-labeled (small panels) antisense (A_–_D) or sense (E) riboprobe for MmEg5. Sympathetic neurons were obtained from superior cervical ganglia of newborn rat pups and were grown for 1, 3, 7, and 14 d. In situ hybridization analyses show that mRNAs encoding Eg5 were expressed at 1, 3, and 7 d. At 14 d, the hybridization signal was similar to that detected at 3 d with the sense riboprobe control. Note also that Eg5 mRNAs are downregulated during in vitro development. Scale bar, 10 μm.
Fig. 5.
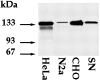
Western blot analyses using a polyclonal antibody against Eg5 on extracts prepared from cultured cells. Western blot analyses were performed on samples extracted from rat cultured sympathetic neurons (SN) at 3 d using an affinity-purified polyclonal antibody raised against a region of the motor domain of HsEg5 that is highly conserved in MmEg5. The mitotic form of the Eg5 protein focuses as a 135 kDa band in CHO, HeLa, and neuroblastoma (N2a) cells, used as positive controls. HeLa cells also show a minor 130 KDa band. The 135 kDa protein is also expressed in postmitotic sympathetic neurons. _Arrows_indicate protein ladder (Life Technologies).
Fig. 6.
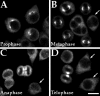
The polyclonal HsEg5 antibody recognizes MmEg5 protein in neuroblastoma cells. The polyclonal antibody generated from the N-terminal motor region of the human Eg5 molecule reveals the same distribution of Eg5 protein during different phases of mitosis in mouse neuroblastoma cells as observed in HeLa cells with an antibody directed against the tail region of HsEg5 (Blangy et al., 1995).Arrows indicate interphase cells in various panels. Each panel also shows one or more cell in a particular stage of mitosis.A, Prophase; B, Metaphase;C, Anaphase; D, Telophase. Scale bar, 10 μm.
Fig. 7.
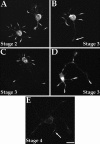
Immunofluorescence analyses on the distribution of Eg5 in cultured rat hippocampal neurons at early stages of development. At stage 2 (A), the protein is localized within cell bodies and within most minor processes. Most typically, it is present at the tips of the minor processes, but sometimes along their lengths. At stage 3, the protein is still present within the cell body and minor processes. In some axons, the protein was no longer observed at the distal tip of the process (B,arrow). In most cases, the protein was observed at the distal tips of the early axon (C, D) and within branches of the axons (D). At stage 4 (E), protein levels were significantly diminished throughout the neuron. Very low levels of protein were sometimes detected at dendrite tips (arrow). Scale bar, 10 μm.
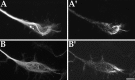
Fig. 8.
Immunofluorescence analyses on the distribution of Eg5 in cultured rat hippocampal neurons at stage 5 of development. Shown are stage 5 hippocampal cultures double-immunostained for β-tubulin in A_–_C to reveal cellular morphology and in _A_′–_C_′ to show Eg5 distribution. Eg5 protein levels are significantly higher than at stage 4. The protein is localized within the tips of dendrites (_A_′), as well as within newly forming sprouts of dendrites (_B_′, _C_′). Scale bar, 5.5 μm.
Fig. 9.
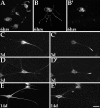
Immunofluorescence analyses on the distribution of Eg5 in developing cultured rat sympathetic neurons. A,B, and _B_′ show neurons cultured for 6 hr.A shows that Eg5 is present within cell bodies, lamellipodia, short processes resulting from coalescence of lamellipodia, and within the distal tips of early axons.B is a β-tubulin double-stain to reveal morphology._B_′ shows that Eg5 is present within the cell bodies and distal tips of somewhat longer axons. The remaining panels show older cultures (3 and 14 d) double-immunostained for a dendrite-enriched neurofilament protein in C_–_E to reveal morphology and for Eg5 in _C_′–_E_′. At 3 d, Eg5 is concentrated at the dendrite tip (_C_′,_D_′) as well as in dendritic branches (_D_′). At 14 d, Eg5 is still detected in the cell body, but is no longer concentrated at the dendrite tip (_E_′). Scale bar: A, 15 μm;_B_–_E_′, 10 μm.
Fig. 10.
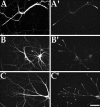
Immunofluorescence analyses on the distribution of Eg5 in cultured hamster cortical neurons. Shown are hamster cortical neurons double-immunostained for β-tubulin in A and_B_ and for Eg5 in _A_′ and_B_′. Eg5 immunostain colocalizes with a subpopulation of microtubule polymer within the growth cone. Scale bar, 13 μm.
Similar articles
- Expression of the mitotic motor protein CHO1/MKLP1 in postmitotic neurons.
Ferhat L, Kuriyama R, Lyons GE, Micales B, Baas PW. Ferhat L, et al. Eur J Neurosci. 1998 Apr;10(4):1383-93. doi: 10.1046/j.1460-9568.1998.00159.x. Eur J Neurosci. 1998. PMID: 9749792 - Expression of the mitotic kinesin Kif15 in postmitotic neurons: implications for neuronal migration and development.
Buster DW, Baird DH, Yu W, Solowska JM, Chauvière M, Mazurek A, Kress M, Baas PW. Buster DW, et al. J Neurocytol. 2003 Jan;32(1):79-96. doi: 10.1023/a:1027332432740. J Neurocytol. 2003. PMID: 14618103 - NEK7 regulates dendrite morphogenesis in neurons via Eg5-dependent microtubule stabilization.
Freixo F, Martinez Delgado P, Manso Y, Sánchez-Huertas C, Lacasa C, Soriano E, Roig J, Lüders J. Freixo F, et al. Nat Commun. 2018 Jun 13;9(1):2330. doi: 10.1038/s41467-018-04706-7. Nat Commun. 2018. PMID: 29899413 Free PMC article. - Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport.
Tas RP, Chazeau A, Cloin BMC, Lambers MLA, Hoogenraad CC, Kapitein LC. Tas RP, et al. Neuron. 2017 Dec 20;96(6):1264-1271.e5. doi: 10.1016/j.neuron.2017.11.018. Epub 2017 Nov 30. Neuron. 2017. PMID: 29198755 Free PMC article. Review. - The role of motor proteins in establishing the microtubule arrays of axons and dendrites.
Baas PW. Baas PW. J Chem Neuroanat. 1998 Jun;14(3-4):175-80. doi: 10.1016/s0891-0618(98)00012-x. J Chem Neuroanat. 1998. PMID: 9704896 Review.
Cited by
- Mini-review: Microtubule sliding in neurons.
Guha S, Patil A, Muralidharan H, Baas PW. Guha S, et al. Neurosci Lett. 2021 May 14;753:135867. doi: 10.1016/j.neulet.2021.135867. Epub 2021 Apr 1. Neurosci Lett. 2021. PMID: 33812935 Free PMC article. Review. - KIFC1 Regulates the Trajectory of Neuronal Migration.
Muralidharan H, Guha S, Madugula K, Patil A, Bennison SA, Sun X, Toyo-Oka K, Baas PW. Muralidharan H, et al. J Neurosci. 2022 Mar 16;42(11):2149-2165. doi: 10.1523/JNEUROSCI.1708-21.2022. Epub 2022 Jan 19. J Neurosci. 2022. PMID: 35046122 Free PMC article. - Inhibition of kinesin-5 improves regeneration of injured axons by a novel microtubule-based mechanism.
Baas PW, Matamoros AJ. Baas PW, et al. Neural Regen Res. 2015 Jun;10(6):845-9. doi: 10.4103/1673-5374.158351. Neural Regen Res. 2015. PMID: 26199587 Free PMC article. Review. - In vitro antitumor activity, molecular dynamics simulation, DFT study, ADME prediction, and Eg5 binding of enastron analogues.
Bouzina A, Bouone YO, Sekiou O, Aissaoui M, Ouk TS, Djemel A, Mansouri R, Ibrahim-Ouali M, Bouslama Z, Aouf NE. Bouzina A, et al. RSC Adv. 2023 Jun 28;13(28):19567-19584. doi: 10.1039/d3ra02904b. eCollection 2023 Jun 22. RSC Adv. 2023. PMID: 37388149 Free PMC article. - KIF11 is required for proliferation and self-renewal of docetaxel resistant triple negative breast cancer cells.
Jiang M, Zhuang H, Xia R, Gan L, Wu Y, Ma J, Sun Y, Zhuang Z. Jiang M, et al. Oncotarget. 2017 Sep 8;8(54):92106-92118. doi: 10.18632/oncotarget.20785. eCollection 2017 Nov 3. Oncotarget. 2017. PMID: 29190901 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases