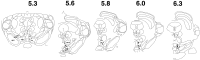Electrophysiological characterization of GABAergic neurons in the ventral tegmental area - PubMed (original) (raw)
Electrophysiological characterization of GABAergic neurons in the ventral tegmental area
S C Steffensen et al. J Neurosci. 1998.
Abstract
GABAergic neurons in the ventral tegmental area (VTA) play a primary role in local inhibition of mesocorticolimbic dopamine (DA) neurons but are not physiologically or anatomically well characterized. We used in vivo extracellular and intracellular recordings in the rat VTA to identify a homogeneous population of neurons that were distinguished from DA neurons by their rapid-firing, nonbursting activity (19.1 +/- 1.4 Hz), short-duration action potentials (310 +/- 10 microseconds), EPSP-dependent spontaneous spikes, and lack of spike accommodation to depolarizing current pulses. These non-DA neurons were activated both antidromically and orthodromically by stimulation of the internal capsule (IC; conduction velocity, 2.4 +/- 0.2 m/sec; refractory period, 0.6 +/- 0.1 msec) and were inhibited by stimulation of the nucleus accumbens septi (NAcc). Their firing rate was moderately reduced, and their IC-driven activity was suppressed by microelectrophoretic application or systemic administration of NMDA receptor antagonists. VTA non-DA neurons were recorded intracellularly and showed relatively depolarized resting membrane potentials (-61.9 +/- 1.8 mV) and small action potentials (68.3 +/- 2.1 mV). They were injected with neurobiotin and shown by light microscopic immunocytochemistry to be multipolar cells and by electron microscopy to contain GABA but not the catecholamine-synthesizing enzyme tyrosine hydroxylase (TH). Neurobiotin-filled dendrites containing GABA received asymmetric excitatory-type synapses from unlabeled terminals and symmetric synapses from terminals that also contained GABA. These findings indicate that VTA non-DA neurons are GABAergic, project to the cortex, and are controlled, in part, by a physiologically relevant NMDA receptor-mediated input from cortical structures and by GABAergic inhibition.
Figures
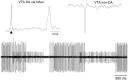
Fig. 1.
Extracellular electrophysiological characterization of dopamine and nondopamine neurons in the ventral tegmental area. Top, Unfiltered recordings of a VTA DA neuron evoked by stimulation of the NAcc (left) and of a spontaneous non-DA neuron (right) are shown. Calibration bar applies to both. VTA DA neurons were slow-firing (<1 Hz), bursting neurons that were driven by NAcc stimulation with spike durations of >500 μsec (arrow indicates NAcc stimulus artifact). VTA non-DA neurons were relatively fast-firing, nonbursting cells that evinced negative-going spikes and were characterized by spike durations of <500 μsec. VTA non-DA neurons were not driven by NAcc stimulation. Bottom, Under halothane anesthesia, VTA non-DA neurons evinced pronounced and persistent phasic activity as demonstrated by the two simultaneously recorded VTA non-DA neurons in the filtered trace.
Fig. 2.
Recording sites of VTA nondopamine neurons. The neuropil surrounding extracellularly recorded VTA non-DA neurons was stained with pontamine sky blue after electrophysiological evaluation. VTA non-DA neurons (filled circles) were localized along the rostrocaudal and dorsoventral extent of the VTA [shown here at 5.3, 5.6, 5.8, 6.0, and 6.3 mm from bregma (Paxinos and Watson, 1986)]; however, they tended to be encountered in clusters from 200 to 1000 μm dorsal to DA neurons. ip, Interpeduncular nucleus; ml, medial lemniscus;pn, paranigral nucleus; rmc, red nucleus, magnocellularis; snc, substantia nigra pars compacta;snr, substantia nigra pars reticulata;vta, ventral tegmental area.
Fig. 3.
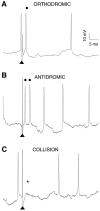
VTA nondopamine neuron reciprocal input to cortex: extracellular recordings. A, Stimulation of the IC elicits a VTA non-DA spike (filled circle). B, A spontaneous spike that precedes IC stimulation extinguishes the driven spike. Calibration bar in_A_ applies to B. The_asterisk_ signifies where the driven spike would have occurred had there been no collision. C, Stimulation of the IC also elicits orthodromic VTA non-DA spikes and produces a period of inhibition of an orthodromic spike that extends to 20 msec (C) when tested with equipotent paired stimuli at a 2× threshold stimulus level. D, IC stimulation produces a period of superexcitability for an antidromic spike when tested with equipotent paired stimuli at a 0.5× threshold stimulus level. Arrows indicate IC stimulus artifacts. The time between arrows or the interstimulus interval (ISI) in C and_D_ is indicated in each graph.
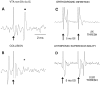
Fig. 4.
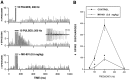
Local application of NMDA receptor antagonists attenuate spontaneous and cortical input to VTA nondopamine neurons.A, The rate meter above shows the firing rate of a VTA non-DA neuron before and after _in situ_microelectrophoretic application of the NMDA antagonist APV (horizontal gray bar). APV significantly decreased the VTA non-DA firing rate. B, Microelectrophoretic application of APV markedly decreased the occurrence of orthodromic VTA non-DA spikes evoked by stimulation of the IC. _Asterisks_indicate significance level (p < 0.001).
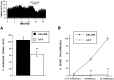
Fig. 5.
Systemic administration of NMDA receptor antagonists attenuate frequency-dependent multiple spike discharges evoked by stimulation of cortical input to VTA nondopamine neurons.A, These peristimulus spike histograms demonstrate the effects of high-frequency stimulation of the IC on VTA non-DA spike discharges. Each histogram represents 10 cumulated epochs of IC stimulation (0.1 Hz; bin width, 2 msec). Stimulation of the IC with 10 pulses at 400 Hz did not evoke spike discharges but produced a 100 msec period of inhibition of spontaneous firing (top), whereas the same number of pulses at 200 Hz elicited multiple spike discharges that occurred with latencies nearly an order of magnitude greater than the single spike latency of 2–3 msec (middle). The inset shows a representative filtered recording of a VTA non-DA neuron after high-frequency stimulation (200 Hz; 10 pulses) of the IC. Systemic administration of the NMDA receptor antagonist MK-801 markedly suppressed the multiple discharging of this VTA non-DA neuron (bottom). The horizontal bar in each histogram represents the stimulus train. B, Summary of the VTA non-DA spike discharges produced by high-frequency IC stimulation shows that the number of spike discharges is a function of the frequency of stimuli. For all frequencies, the number of pulses and the number of epochs were held constant at 10 while varying the interval between pulses. Although 50 and 400 Hz evoked little or no spike discharges, 200 Hz markedly and 100 Hz moderately increased discharging. Systemic administration of MK-801 significantly reduced VTA non-DA spike discharging produced by IC stimulation across frequencies. Asterisks indicate significance level (p < 0.001).
Fig. 6.
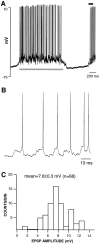
VTA nondopamine neuron spontaneous activity is dependent on synaptic input. A, A representative 2.5 sec_trace_ of a VTA non-DA neuron recorded intracellularly in a halothane-anesthetized rat is shown. The resting membrane potential of this VTA non-DA neuron was −61 mV, and the spike amplitude was 70.5 mV. This and all other VTA non-DA neurons were characterized by pronounced phasic ON and OFF activity under halothane anesthesia (see Fig. 1). The ON phase of activity was accompanied by a 10.6 mV depolarization. B, The time base during the period indicated by the horizontal black line in_A_ is expanded to show the individual spikes. An EPSP appeared to precede every spontaneous spike. The voltage axis is the same as that in A. C, The amplitudes of each EPSP during the period indicated by the horizontal gray line in A were measured and plotted in the histogram and were characterized by a normal distribution of spontaneous EPSP amplitudes. The mean EPSP amplitude was 7.6 ± 0.3 mV (n = 68).
Fig. 7.
VTA nondopamine neuron reciprocal input to cortex: intracellular recordings. A, Similar to that in the extracellular recordings in Figure 3, stimulation of the IC (arrowheads indicate stimulus artifact) consistently evoked intracellularly recorded VTA non-DA spikes at short latency. In this example, the driven spike (filled circle) appeared to be orthodromic because a spontaneous spike occurred within a spontaneous-spike to driven-spike interval that was less than twice the latency of the driven spike plus the refractory period. In addition, a small EPSP preceded the driven spike. The spike at the_far right_ was a spontaneous spike and was not time-locked to successive stimuli. B, This VTA non-DA spike doublet was driven antidromically from the IC. Spike doublets occurred more with intracellular than with extracellular recordings. Note that there is little or no EPSP preceding the doublet of short latency spikes even though the spontaneous spikes are accompanied by EPSPs. C, The doublet of IC-driven spikes is extinguished by a spontaneous spike that occurs within an interval that was less than twice the latency of the first driven spike plus the refractory period. The asterisk indicates where the spikes would have occurred in the absence of collision. The calibration bars in A apply to B and_C_.
Fig. 8.
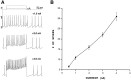
Lack of accommodation in VTA nondopamine neurons. This neuron had a resting membrane potential of −64 mV.A, Increasing levels of depolarizing current produced multiple VTA non-DA spiking. Current steps are shown_above_ the traces. The calibration bar (top) is the same for all traces.B, Summary of the input/output response for spiking produced by depolarizing current demonstrates the lack of accommodation of VTA non-DA neurons.
Fig. 9.
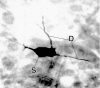
Neurobiotin labeling of VTA nondopamine neurons_in vivo_. Light micrograph of a neurobiotin-labeled non-DA neuron in the VTA. This is the same neuron studied electrophysiologically in Figure 8. This neuron was characteristic of all neurons identified electrophysiologically as VTA non-DA neurons and was multipolar in shape with few dendritic processes (D) branching from its soma (S).
Fig. 10.
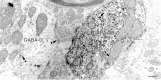
VTA nondopamine neurons contain GABA immunoreactivity. Electron micrograph of the neuron in Figure 9shows two dendrites (GABA-D and_GABA/NB-D_) that contain immunogold-silver particles for GABA (arrows). GABA/NB-D also contains peroxidase reaction product for neurobiotin, indicative of the physiologically characterized non-DA neuron. The filled and nonfilled GABA dendrites are linked by two common axon unlabeled terminals (UTs), which appear to form asymmetric synapses. The gold-silver particles for GABA are sparse but are indicative of specific labeling because there is virtually a total absence of spurious particles in the tissue. Scale bar, 0.4 μm.
Similar articles
- Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons.
Carr DB, Sesack SR. Carr DB, et al. J Neurosci. 2000 May 15;20(10):3864-73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. J Neurosci. 2000. PMID: 10804226 Free PMC article. - Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons.
Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ. Steffensen SC, et al. Exp Neurol. 2006 Nov;202(1):139-51. doi: 10.1016/j.expneurol.2006.05.023. Epub 2006 Jun 30. Exp Neurol. 2006. PMID: 16814775 - Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels.
Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, Allison DW. Steffensen SC, et al. Eur J Neurosci. 2008 Nov;28(10):2028-40. doi: 10.1111/j.1460-9568.2008.06479.x. Eur J Neurosci. 2008. PMID: 19046384 Free PMC article.
Cited by
- In silico Hierarchical Clustering of Neuronal Populations in the Rat Ventral Tegmental Area Based on Extracellular Electrophysiological Properties.
Di Miceli M, Husson Z, Ruel P, Layé S, Cota D, Fioramonti X, Bosch-Bouju C, Gronier B. Di Miceli M, et al. Front Neural Circuits. 2020 Aug 13;14:51. doi: 10.3389/fncir.2020.00051. eCollection 2020. Front Neural Circuits. 2020. PMID: 32903825 Free PMC article. - Quantitative unit classification of ventral tegmental area neurons in vivo.
Li W, Doyon WM, Dani JA. Li W, et al. J Neurophysiol. 2012 May;107(10):2808-20. doi: 10.1152/jn.00575.2011. Epub 2012 Feb 29. J Neurophysiol. 2012. PMID: 22378178 Free PMC article. - Modulation of ligand-gated ion channels by antidepressants and antipsychotics.
Rammes G, Rupprecht R. Rammes G, et al. Mol Neurobiol. 2007 Apr;35(2):160-74. doi: 10.1007/s12035-007-0006-1. Mol Neurobiol. 2007. PMID: 17917105 Review. - Ventral tegmental area dopamine and GABA neurons: Physiological properties and expression of mRNA for endocannabinoid biosynthetic elements.
Merrill CB, Friend LN, Newton ST, Hopkins ZH, Edwards JG. Merrill CB, et al. Sci Rep. 2015 Nov 10;5:16176. doi: 10.1038/srep16176. Sci Rep. 2015. PMID: 26553597 Free PMC article. - Phospholipase Cgamma in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors.
Bolaños CA, Perrotti LI, Edwards S, Eisch AJ, Barrot M, Olson VG, Russell DS, Neve RL, Nestler EJ. Bolaños CA, et al. J Neurosci. 2003 Aug 20;23(20):7569-76. doi: 10.1523/JNEUROSCI.23-20-07569.2003. J Neurosci. 2003. PMID: 12930795 Free PMC article.
References
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. - PubMed
- Beart PM, McDonald D. Neurochemical studies of the mesolimbic dopaminergic pathway: somatodendritic mechanisms and GABAergic neurones in the rat ventral tegmentum. J Neurochem. 1980;34:1622–1629. - PubMed
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. - PubMed
- Bunney BS, Aghajanian GK, Roth RH. Comparison of effects of l-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurons. Nat New Biol. 1973;245:123–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AA10075/AA/NIAAA NIH HHS/United States
- R01 DA004600/DA/NIDA NIH HHS/United States
- R01 MH040342/MH/NIMH NIH HHS/United States
- R01 AA013666/AA/NIAAA NIH HHS/United States
- DA08301/DA/NIDA NIH HHS/United States
- DA04600/DA/NIDA NIH HHS/United States
- R37 MH040342/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous