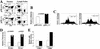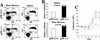CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis - PubMed (original) (raw)
CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis
D S Franklin et al. Genes Dev. 1998.
Abstract
INK4 and CIP/KIP are two distinct families of cyclin-dependent kinase (CDK) inhibitors implicated in mediating a wide range of cell growth control signals. We have created p18(INK4c)-deficient mice. These mice develop gigantism and widespread organomegaly. The pituitary gland, spleen, and thymus are disproportionately enlarged and hyperplastic. T and B lymphocytes develop normally in p18-deficient mice, but both exhibit increased cellularity and a higher proliferative rate upon mitogenic stimulation. Loss of p18, like that of p27, but not other CDK inhibitor genes, leads to a gradual progression from intermediate lobe pituitary hyperplasia in young mice to an adenoma by 10 months of age with a nearly complete penetrance. Mice lacking both p18 and p27, like mice chimeric for Rb deficiency, invariably died from pituitary adenomas by 3 months. Hence, p18 and p27 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis, likely by controlling the function of Rb.
Figures
Figure 1
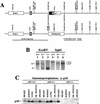
Targeted disruption of p18INK4c locus. (A) Genomic structure of the mouse INK4c locus. The mouse INK4c locus contains three exons. Coding region is shown by black boxes. The relative positions of restriction sites and translation initiation and termination codons are indicated. p18 was disrupted by replacement of the 2-kb _Eco_RI fragment containing exon 3 with the neor selectable marker. The 4.6- and 1.9-kb genomic fragments used for homologous recombination and the probe fragment used for Southern analysis are indicated. (B) Southern blot analysis of the p18 locus. Genomic DNA from p18+/+ (lanes 1,4), p18+/− (lanes 2,5) and p18−/− (lanes 3,6) mice was digested with restriction enzymes _Eco_RV (lanes 1–3) or _Sph_I (lanes 4–6) and hybridized with a p18 probe (see A). Molecular size markers are indicated. (C) p18 protein expression in mouse tissues. Total cell lysates were prepared from several p18+/+, p18+/−, and p18−/− tissues. Expression of p18 protein was determined by IP–Western blot analysis with antibodies specific for p18. The p18 protein is indicated.
Figure 2
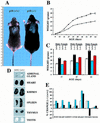
p18-deficient mice exhibit body size increase and organomegaly. (A) Wild-type (agouti, left) and p18-deficient (black, right) mice at 4 weeks of age. Size is indicated in centimeters. (B) Growth curve of p18-deficient (█) and wild-type (□) mice. Both genotypes (nine each) were weighed from age 9 to 63 days. Standard error bars are indicated. (C) Body weight comparison of male and female p18-deficient and wild-type mice. Data were taken from B for days 33, 45, and 63 and replotted according to gender: wild-type males (open bars), null males (black bars), wild-type females (blue bars), and null females (red bars). (D) Organomegaly in p18−/− mice. Gross appearance of adrenal gland, heart, kidney, spleen, thymus, and testis are shown for p18+/+ and p18−/− animals. (E) Increase of body and individual organ weights in p18−/− mice relative to wild-type littermates. p18+/+ and p18−/− animals were weighed at ages 1 month (open bars), 2 months (blue bars) and 3 months (black bars). Heart, kidney, liver, spleen, thymus, and testis from these mice were isolated and weighed. The data are plotted as relative body and individual organ weight differences of the p18−/− animal compared with the p18+/+ animals.
Figure 3
Hyperproliferative response of p18-deficient T lymphocytes to mitogenic signals. (A) Flow cytometric analysis of T lymphocytes. Cells from the thymus and lymph nodes were isolated from 1-month-old animals and CD4/CD8 profiles were determined. (B) Increased thymus cellularity. Thymocytes were isolated from 1-month-old wild-type (open bar) or p18-null (solid bar) animals, and cell counts were obtained for each organ. (C) Apoptosis assay of thymocytes in response to CD3. One-month-old wild-type (left) and p18-null (right) animals were injected intraperitoneally with dexamethasone for 12 hr. Early apoptotic cells were assayed by TdT end labeling and TdT–FITC FACS analysis. The percentage of apoptotic cells is indicated with standard error. (D) In vitro and in vivo proliferation of lymph node cells. For the in vitro assay, lymph node cells were isolated from 1-month-old wild-type (open bar) or p18-null (solid bar) animals and cultured in vitro. After ConA mitogenic stimulation, cells were pulsed with [3H]thymidine. For in vivo stimulation, wild-type (open bar) and p18-null (solid bar) animals were injected intraperitoneally with ConA for 12 hr. Cells were isolated from lymph nodes and immediately pulsed with [3H]thymidine. S-phase populations were determined by measurement of 3H incorporation. (E) CDK4 and CDK6 kinase assay. Twelve hours after in vivo intraperitoneal ConA injection, CDK4 and CDK6 complexes were immunoprecipitated from wild-type (open bars) or p18−/− (solid bars) lymph nodes and assayed for their kinase activity with a GST–Rb fusion protein as substrate.
Figure 4
Hyperproliferative response of p18-deficient B lymphocytes to mitogenic signals. (A) Flow cytometric analysis of B lymphocytes. Cells from the bone marrow and spleen were isolated from 2-month-old animals, and IgM and B220 profiles were determined. (B) Increased cellularity. Bone marrow and spleen were isolated from 2-month-old wild-type (open bars) or p18-null (solid bars) animals, and cell counts were obtained for each organ. (C) Mitogenic stimulation of B lymphocytes. Resting B lymphocytes were purified from p18+/+ (▵) and p18−/− (█) spleens. A total of 1.5 × 105 cells/ml were cultured in vitro in the presence of CD40 ligand [CD40L (▵) p18+/+; (□) p18−/−] or in the presence of CD40L and IL-6 [(▴) p18+/+; (█) p18−/−]. Viable cells were determined every 24 hr by trypan blue dye exclusion and expressed as viable cells per milliliter. (D) Apoptosis assay of stimulated B cells. Resting B-lymphocyte populations were purified from wild-type and p18-null animals and cultured in the presence of CD40L with or without IL-6. At day 5, cells were analyzed for early apoptosis with FITC-conjugated annexin V antibody and PI double staining. Other time points were also normal between wild-type and p18-null cells.
Figure 4
Hyperproliferative response of p18-deficient B lymphocytes to mitogenic signals. (A) Flow cytometric analysis of B lymphocytes. Cells from the bone marrow and spleen were isolated from 2-month-old animals, and IgM and B220 profiles were determined. (B) Increased cellularity. Bone marrow and spleen were isolated from 2-month-old wild-type (open bars) or p18-null (solid bars) animals, and cell counts were obtained for each organ. (C) Mitogenic stimulation of B lymphocytes. Resting B lymphocytes were purified from p18+/+ (▵) and p18−/− (█) spleens. A total of 1.5 × 105 cells/ml were cultured in vitro in the presence of CD40 ligand [CD40L (▵) p18+/+; (□) p18−/−] or in the presence of CD40L and IL-6 [(▴) p18+/+; (█) p18−/−]. Viable cells were determined every 24 hr by trypan blue dye exclusion and expressed as viable cells per milliliter. (D) Apoptosis assay of stimulated B cells. Resting B-lymphocyte populations were purified from wild-type and p18-null animals and cultured in the presence of CD40L with or without IL-6. At day 5, cells were analyzed for early apoptosis with FITC-conjugated annexin V antibody and PI double staining. Other time points were also normal between wild-type and p18-null cells.
Figure 5
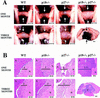
Pituitary tumorigenesis in p18−/− mice. Pituitaries from p18+/+ (healthy 10-month-old, A–C), p18−/− (healthy 10-month-old with hyperplasia, D–F) and p18−/− (debilitated 9.5-month-old with adenoma, G–I). (A,D,G) Comparative sizes of pituitaries. Arrows indicate pituitaries. Bar, 1 mm. (B,E,H) Histological staining of the pituitaries. Cross sections of pituitaries of p18+/+ (B) and p18−/− (E) and mid-sagittal section of p18−/− mice (H) were stained with hematoxylin and eosin. (A) Anterior lobe; (I) intermediate lobe; [I(T)] intermediate lobe tumor; and (N) neurohypophysis. (*) Hyperplastic nodule. (Black bars) Width of the intermediate lobe. Size bars, ∼70 μm for B and E and 1 mm for H. (C,F,I) ACTH staining of pituitaries. Cross-section of pituitaries of control p18+/+ (C) and p18−/− (F) and mid-sagittal section of p18−/− mice (I) were stained for ACTH. Abbreviations and symbols are as in other panels. Size bars, ∼70 μm for C and F and 1 mm for I.
Figure 6
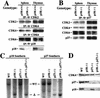
p27 and p27–CDK complexes in p18-deficient mice. (A) Expression of G1 CDK2, CDK4, and CDK6 is unchanged in p18-null cells. Total protein lysates were prepared from thymuses and spleens of wild-type or p18-null mice. The levels of the three CDK and p18 proteins were determined by IP–Western blot analysis with the indicated antisera. (B) p27–CDK complexes were not affected in p18-null cells. Total protein lysates prepared from thymuses and spleens of wild-type or p18-null mice were precipitated with anti-p27 antibody, and the level of p27 associated CDK proteins was determined by immunoblotting. (C) Generation of p18−/−/p27−/− double-mutant mice. Southern blot analysis of the p18 and p27 loci. Genomic DNA from mice with four different genotypes was digested with _Eco_RV (for p18) or _Eco_RI (for p27) and hybridized with a p18 and a p27 probe as indicated. (D) Expression of p18 and p27 proteins in mice pituitary. Equal amounts of total cell lysates prepared from pituitary tissues of mice with four different genotypes were resolved by SDS-PAGE. p18 and p27 protein levels were determined by immunoblotting. The level of CDK4 protein remains relatively constant in different genetic backgrounds and was used as an additional control.
Figure 7
Pituitary tumorigenesis in p18−/−/p27−/− mice. (A) Pituitaries from wild-type, p18−/−, p27−/−, and p18−/−/p27−/− mice at 1 and 3 months were compared. (Arrows) Pituitaries. Bars, 1 mm. Note the different size bar (also 1 mm) for the pituitary from the 3-month-old p18−/−/p27−/− mouse. (B) Histological staining of wild-type, p18−/−, p27−/−, and p18−/−/p27−/− pituitaries. Cross sections or mid-sagittal sections of pituitaries of the four different genotypes were stained with hematoxylin and eosin. (A) Anterior lobe; (I) intermediate lobe; and (N) neurohypophysis. (Black bars) Width of the intermediate lobe. Size bars, 70 mm, except for the pituitary from the 3-month-old p18−/−/p27−/− mouse (1 mm).
Figure 8
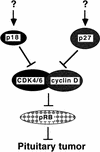
Suppression of pituitary tumorigenesis by p18 and p27. p18 and p27 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. The upstream regulatory signals leading to the activation of p18 and p27 are unknown. Rb is suggested to be the common target of p18 and p27 function on the basis of the observations that the biochemical functions of p18 and p27 are to inhibit CDK activity regulating pRb function and that chimeric Rb−/− mice and p18−/−/p27−/− double-deficient mice develop pituitary tumors at essentially the same rate.
References
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. - PubMed
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai L-H, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. - PubMed
- Guan K-L, Jenkins CW, Li Y, Nichols MA, Wu X, O’Keefe CL, Matera AG, Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes & Dev. 1994;8:2939–2952. - PubMed
- Hu N, Gutsmann A, Herbert DC, Bradley A, Lee W-H, Lee EY-HP. Heterzygous Rb-1D20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994;9:1021–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA068377/CA/NCI NIH HHS/United States
- AR43455/AR/NIAMS NIH HHS/United States
- AR44580/AR/NIAMS NIH HHS/United States
- R01 CA065572/CA/NCI NIH HHS/United States
- CA65572/CA/NCI NIH HHS/United States
- R01 AI041356/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous