A single binding site for dilysine retrieval motifs and p23 within the gamma subunit of coatomer - PubMed (original) (raw)
A single binding site for dilysine retrieval motifs and p23 within the gamma subunit of coatomer
C Harter et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Coatomer, the major component of the coat of COPI transport vesicles, binds both to the dilysine motif of resident membrane proteins of the endoplasmic reticulum and to the cytoplasmic domain of p23, a major type I membrane protein of COPI vesicles. Using a photocrosslinking approach, we find that under native conditions a peptide analogous to the cytoplasmic domain of p23 interacts with coatomer exclusively through its gamma subunit and shares its binding site with a KKXX retrieval motif. However, upon dissociation of coatomer, interaction with various subunits, including an alpha-, beta'-, epsilon-COP subcomplex, of the photoreactive peptide is observed. We suggest that, under physiological conditions, interaction of coatomer with both endoplasmic reticulum retrieval motifs and the cytoplasmic domain of p23 is mediated by gamma-COP.
Figures
Figure 1
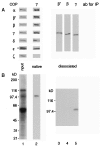
Peptides used in this study. (A) Peptide sequences of p23wt, p23A, p23AS, and Wbp1p. p23wt represents the cytoplasmic domain of p23 known to bind coatomer. p23A binds coatomer less efficiently than the wild-type peptide, whereas the peptide p23AS has lost this capability. Wbp1p represents the cytoplasmic domain of a subunit of the yeast N-oligosaccharyltransferase complex and contains a characteristic KKXX ER-retrieval motif also known to interact with coatomer. (B) Immobilized, photoreactive p23wt peptide (125I-F*-p23wt). The natural Phe at position −8 was replaced by the photoreactive analogue
l
-4-[3-(trifluoromethyl-3_H_-diazirin-3-yl)]phenylalanine (F*, Tmd-Phe). F*-p23wt peptide was immobilized by coupling to thiopropyl-Sepharose by a disulfide bond and was radioactively labeled with [125I]iodine.
Figure 2
Photocrosslinking of coatomer with immobilized125I-F*-p23wt. Coatomer was incubated with125I-F*-p23wt Sepharose for 90 min on ice prior to irradiation for 2 min on ice. Lanes 1, total photocrosslinked products. Lanes 2–9, samples immunoprecipitated with antibodies against individual COP proteins or the cytoplasmic domain of p23. (A) Immunoblot. (B) Autoradiogram of_A_. The samples were analyzed by SDS/PAGE, immunoblotting, and autoradiography. COPs were detected with a mixture of antibodies against all seven COPs. Total protein was separated on SDS/7.5–16.5% polyacrylamide gradient gels (lanes 1), α-, β-, β′-, γ-, and δ-COP were separated on 7.5% gels (lanes 2–7); ɛ- and ζ-COP were separated on 15% gels (lanes 8 and 9).
Figure 3
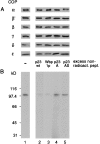
Photocrosslinking of cell lysate with125I-F*-p23wt in solution. CHO cell lysate was incubated with 125I-F*-p23wt in solution for 1 h on ice prior to photoactivation for 2 min on ice. (A) Lane 1, immunoblot of total cell lysate analyzed with a mixture of antibodies against all seven coatomer subunits. The asterisk indicates a cross-reactivity of the anti-ɛ-COP antiserum with a protein present in the lysate. Lane 2, immunoblot analysis of coatomer immunoprecipitated with an anti-γ-COP antibody without prior dissociation. Lanes 3–5, immunoblot analysis of β′-, β-, and γ-COPs individually immunoprecipitated (IP) upon dissociation of coatomer by SDS treatment. Samples were separated on 7.5–16.5% (lanes 1 and 2) or 7.5% polyacrylamide gels (lanes 3–5) in the presence of SDS. (B) Autoradiogram of A.
Figure 4
Peptide competition experiments. CHO lysate was incubated with 125I-F*-p23wt in solution in the absence (lane 1) or presence (lanes 2, 3, 4, and 5) of a 10-fold excess of nonradioactive peptides, and irradiated. Coatomer was immunoprecipitated with an anti-γ-COP antibody. Samples were separated on SDS/7.5–16.5% polyacrylamide gradient gels. (A) Immunoblot with antibodies against α-ɛ-COPs. (B) Autoradiogram of A.
Figure 5
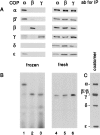
Photocrosslinking of frozen CHO lysate with125I-F*-p23wt in solution. Incubations and photocrosslinking were performed as described in the legend to Fig. 3 with the exception that lysates used for the experiments shown in lanes 1–3 were frozen in liquid nitrogen, stored at −80°C, and thawed shortly before use. (A) Immunoblot. Lane 1, immunoprecipitation of COPs with an antibody against α-COP; lane 2, against β-COP; lane 3, against γ-COP. Lanes 4–6, immunoprecipitations analogous to those shown in lanes 1–3 but from freshly prepared cell lysates. (B) Autoradiogram of_A_. Coatomer as a standard, with COPs visualized by immunoblotting.
Similar articles
- Nonclathrin coat protein gamma, a subunit of coatomer, binds to the cytoplasmic dilysine motif of membrane proteins of the early secretory pathway.
Harter C, Pavel J, Coccia F, Draken E, Wegehingel S, Tschochner H, Wieland F. Harter C, et al. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1902-6. doi: 10.1073/pnas.93.5.1902. Proc Natl Acad Sci U S A. 1996. PMID: 8700856 Free PMC article. - Bimodal interaction of coatomer with the p24 family of putative cargo receptors.
Fiedler K, Veit M, Stamnes MA, Rothman JE. Fiedler K, et al. Science. 1996 Sep 6;273(5280):1396-9. doi: 10.1126/science.273.5280.1396. Science. 1996. PMID: 8703076 - A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding.
Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. Sohn K, et al. J Cell Biol. 1996 Dec;135(5):1239-48. doi: 10.1083/jcb.135.5.1239. J Cell Biol. 1996. PMID: 8947548 Free PMC article. - Targeting to the endoplasmic reticulum by dilysine motifs: role of coat proteins.
Cosson P, Letourneur F. Cosson P, et al. Cold Spring Harb Symp Quant Biol. 1995;60:113-7. doi: 10.1101/sqb.1995.060.01.014. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824383 Review. No abstract available. - COPs regulating membrane traffic.
Kreis TE, Lowe M, Pepperkok R. Kreis TE, et al. Annu Rev Cell Dev Biol. 1995;11:677-706. doi: 10.1146/annurev.cb.11.110195.003333. Annu Rev Cell Dev Biol. 1995. PMID: 8689572 Review.
Cited by
- Secretory protein biogenesis and traffic in the early secretory pathway.
Barlowe CK, Miller EA. Barlowe CK, et al. Genetics. 2013 Feb;193(2):383-410. doi: 10.1534/genetics.112.142810. Genetics. 2013. PMID: 23396477 Free PMC article. Review. - Cell-Sonar, a Novel Method for Intracellular Tracking of Secretory Pathways.
Brockmöller S, Seeger T, Worek F, Rothmiller S. Brockmöller S, et al. Cells. 2024 Aug 29;13(17):1449. doi: 10.3390/cells13171449. Cells. 2024. PMID: 39273021 Free PMC article. - 9Å structure of the COPI coat reveals that the Arf1 GTPase occupies two contrasting molecular environments.
Dodonova SO, Aderhold P, Kopp J, Ganeva I, Röhling S, Hagen WJH, Sinning I, Wieland F, Briggs JAG. Dodonova SO, et al. Elife. 2017 Jun 16;6:e26691. doi: 10.7554/eLife.26691. Elife. 2017. PMID: 28621666 Free PMC article. - Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23.
Gommel DU, Memon AR, Heiss A, Lottspeich F, Pfannstiel J, Lechner J, Reinhard C, Helms JB, Nickel W, Wieland FT. Gommel DU, et al. EMBO J. 2001 Dec 3;20(23):6751-60. doi: 10.1093/emboj/20.23.6751. EMBO J. 2001. PMID: 11726511 Free PMC article. - The C-terminal dilysine motif confers endoplasmic reticulum localization to type I membrane proteins in plants.
Benghezal M, Wasteneys GO, Jones DA. Benghezal M, et al. Plant Cell. 2000 Jul;12(7):1179-201. doi: 10.1105/tpc.12.7.1179. Plant Cell. 2000. PMID: 10899983 Free PMC article.
References
- Rothman J E. Nature (London) 1994;372:55–63. - PubMed
- Waters M G, Serafini T, Rothman J E. Nature (London) 1991;349:248–251. - PubMed
- Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman J E, Wieland F T. Nature (London) 1991;349:215–220. - PubMed
- Duden R, Griffiths G, Frank R, Argos P, Kreis T E. Cell. 1991;64:649–665. - PubMed
- Hosobuchi M, Kreis T, Schekman R. Nature (London) 1992;360:603–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources