HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells - PubMed (original) (raw)
Comparative Study
HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells
N Uchida et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Recent studies have opened the possibility that quiescent, G0/G1 hematopoietic stem cells (HSC) can be gene transduced; lentiviruses (such as HIV type 1, HIV) encode proteins that permit transport of the viral genome into the nucleus of nondividing cells. We and others have recently demonstrated efficient transduction by using an HIV-1-based vector gene delivery system into various human cell types including human CD34(+) cells or terminally differentiated neurons. Here we compare the transduction efficiency of two vectors, HIV-based and murine leukemia virus (MuLV)-based vectors, on untreated and highly purified human HSC subsets that are virtually all in G0/G1. The HIV vector, but not MuLV vector supernatants, transduced freshly isolated G0/G1 HSC from mobilized peripheral blood. Single-step transduction using replication-defective HIV resulted in HSC that expressed the green fluorescent protein (GFP) transgene while retaining their stem cell phenotype; clonal outgrowths of these GFP+ HSC on bone marrow stromal cells fully retained GFP expression for at least 5 weeks. MuLV-based vectors did not transduce resting HSC, as measured by transgene expression, but did so readily when the HSC were actively cycling after culture in vitro for 3 days in a cytokine cocktail. These results suggest that resting HSC may be transduced by lentiviral-based, but not MuLV, vectors and maintain their primitive phenotype, pluripotentiality, and at least in vitro, transgene expression.
Figures
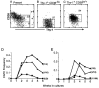
Figure 1
In vitro proliferative potential of CD34+ Thy-1+ Lin− MPB cells further subdivided based on CD38 expression. MPB CD34+ Lin− Thy-1+CD38−/lo and CD38lo/+, and CD34+ Lin− Thy-1− cells were sorted as described. The Thy-1 vs. CD38 profile of CD34+Lin−-gated cells revealed that the CD38 negative cells were highly enriched in Thy-1+ cells and CD38++cells were virtually Thy-1− (A). Reanalysis of CD34+ Lin− Thy-1+CD38−/lo (B) and CD34+Lin− Thy-1+ CD38lo/+(C) populations shows that we could not eliminate “CD38lo ” overlap, although sorting gates were defined to separate CD38− and CD38+ cells. With single-step sorts, the purity of CD38−/lo and CD38lo/+ subsets were 97 ± 1 (SE) % and 91 ± 2%, respectively, by the gates shown. These sorted cells were highly enriched for Thy-1+ expression (99 ± 0.08%, without CD38 gate). The mean fluorescent intensity of the Thy-1 profile on the CD38−/lo subset is consistently brighter (80 ± 8 fluorescence units) than the CD38lo/+ subset (53 ± 3) (P < 0.05). The CAFC frequency of CD34+ Lin− Thy-1+CD38−/lo (•), CD34+Lin− Thy-1+ CD38lo/+ (▴), and CD34+ Lin− Thy-1− (■) MPB cells from two independent experiments are shown in_D_ and E. CAFC frequencies were calculated by linear regression analysis and the χ2 test was performed to validate linear regression analyses. The following CAFC frequencies at week 6 with lower and upper 95% confidence intervals were obtained; in experiment 1 (D) Thy-1+CD38−/lo cells, 1/4 (1/2–1/5); Thy-1+CD38lo/+ cells, 1/10 (1/4–1/24); and Thy-1− cells, undetectable, (UD). In experiment 2 (E), Thy-1+ CD38−/lo cells, 1/9 (1/6–1/13); Thy-1+ CD38lo/+ cells, 1/12 (1/8–1/21); and Thy-1− cells. 1/106 (1/49–1/248).
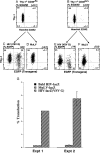
Figure 2
Transgene expression of CD34+Lin− Thy-1+ CD38−/lo and CD38lo/+ cells transduced immediately after the sort. CD34+ Lin− Thy-1+CD38−/lo (A) and CD38lo/+(B) cells were sorted and analyzed for cell cycle status Hoechst 33342 (DNA content) and Thy-1 profile. These cells were transduced by with either HIV-eGFP (VSV-G) or MuLV-eGFP (A-Mo-MLV) by spinoculation for 4 hr without preculture as described in_Materials and Methods_; cells were cultured for 4 days. Five independent experiments revealed that an average of 36 ± 3% of CD34+ Lin− Thy-1+CD38−/lo cells expressed GFP using HIV (VSV-G) (C), whereas virtually no cells expressed GFP (0.5 ± 0.2%) using the MuLV-eGFP (A-Mo-MLV) vector (D) (P< 0.0001). Similarly, an average of 16 ± 2% CD34+Lin− Thy-1+ CD38lo/+ cells expressed GFP using HIV-eGFP (VSV-G) (E), 1 ± 0.5% expressed GFP using MuLV-eGFP(A-Mo-MLV) vector (F) (P < 0.0001). Thy-1+ CD38−/lo cells were transduced by HIV-eGFP(VSV-G) with higher efficiency than Thy-1+CD38lo/+ cells (P < 0.0005). Sorted CD34+ Thy-1+ cells were transduced overnight by nit gravity in the presence into cytokine-containing medium with either MuLV-LacZ(VSV-G), MuLV-lacZ(A-Mo-MLV), ultrafiltrated bald HIV-lacZ, or ultrafiltrated HIV-lacZ(VSV-G) (G). Two days after coculture, fixed cells were stained for β-galactosidase and visually scored for blue color. In Exp. 1, MuLV-lacZ(A-Mo-MLV) was used, and in experiment 2, MuLV-lacZ(VSV-G). The mean and SD of triplicate results are shown for experiment 2. HIV-lacZ (VSV-G) was significantly better (P < 0.001) at transduction when, compared with MuLV-lacZ (VSV-G). The 20-fold difference between HIV and MuLV was reproduced in two other experiments, using different viral supernatant and stem cell preparations. The presence of Vpr, matrix, and integrase mostly facilitate nuclear pore entry of the lentiviral vectors in nondividing cells (51).
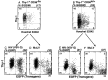
Figure 3
Transduction efficiency of CD34+Lin− Thy-1+ CD38−/lo and CD38lo/+ cells after 3 days in vitro. CD34+ Lin− Thy-1+CD38−/lo and CD38lo/+ cells were sorted and cultured for 3 days as described in Materials and Methods. Hoechst 33342 (DNA content) vs. Thy-1 profile of CD38−/lo (A) and CD38lo/+(B) showed these cells were actively cycling. After 3-days in culture, cells were transduced either with HIV-eGFP (VSV-G) or MuLV-eGFP(A-Mo-MLV) by spinoculation for 4 hr. Cells were cultured for an additional 3–4 days before testing transgene expression. Five independent experiments revealed that a high but equal fraction of CD34+ Lin− Thy-1+CD38−/lo cells resulted in an average of 34 ± 4% cells expressing GFP using HIV-eGFP (VSV-G) (C) compared with MuLV-eGFP(A-Mo-MLV) vector (32 ± 1%) (D) (P = 0.6; no statistically significant difference). Similarly, CD34+ Lin− Thy-1+CD38lo/+ cells resulted in average of 27 ± 3% expressing GFP using HIV (VSV-G) (E) as well as by MuLV vector (33 ± 3%) (F) (P = 0.2, no significant difference).
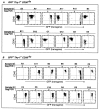
Figure 4
GFP expression of progeny from single cell derived HSC after 5–6 wk of in vitro culture. Freshly isolated CD34+ Lin− Thy-1+CD38−/lo and CD38lo/+ cells were transduced by HIV-eGFP (VSV-G), cultured for 3–4 days and resorted based on GFP expression as described in Materials and Methods. After 5–6 weeks of SyS-1 coculture, proliferating human cells were scored and harvested to evaluate eGFP expressions. Human CD45 vs. GFP expression on progeny of single cells derived GFP− Thy-1+ CD38−/lo(A) and GFP+ Thy-1+CD38−/lo (B) from the first 12 samples harvested were shown. These samples were also tested for the presence of Gag sequences by DNA-PCR analysis as indicated. In some samples, the down-regulation of CD45 expression was observed. It has been shown that thrombopoietin could induce differentiation of cells of the megakaryocyte lineage, and CD45 expression is down regulated as cells differentiate. In this experiment, the frequency of cells contributing to detectable levels of hematopoietic cell proliferation was 1 in 2.2 cells in GFP− Thy-1+ CD38−/loand 1 in 5.9 cells from GFP+Thy-1+CD38−/lo population. The frequency of CAFC was 1 in 3.9 cells in GFP− Thy-1+ CD38−/loand 1 in 21.6 cells from GFP+ Thy-1+CD38−/lo population.
Similar articles
- Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1-based lentiviral vectors.
Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, Haas DL, Xu D, Stripecke R, Naldini L, Kohn DB, Crooks GM. Case SS, et al. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):2988-93. doi: 10.1073/pnas.96.6.2988. Proc Natl Acad Sci U S A. 1999. PMID: 10077624 Free PMC article. - Stable transduction with lentiviral vectors and amplification of immature hematopoietic progenitors from cord blood of preterm human fetuses.
Luther-Wyrsch A, Costello E, Thali M, Buetti E, Nissen C, Surbek D, Holzgreve W, Gratwohl A, Tichelli A, Wodnar-Filipowicz A. Luther-Wyrsch A, et al. Hum Gene Ther. 2001 Mar 1;12(4):377-89. doi: 10.1089/10430340150504000. Hum Gene Ther. 2001. PMID: 11242530 - Development of HIV vectors for anti-HIV gene therapy.
Poeschla E, Corbeau P, Wong-Staal F. Poeschla E, et al. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11395-9. doi: 10.1073/pnas.93.21.11395. Proc Natl Acad Sci U S A. 1996. PMID: 8876146 Free PMC article. Review. - In Vivo Hematopoietic Stem Cell Transduction.
Richter M, Stone D, Miao C, Humbert O, Kiem HP, Papayannopoulou T, Lieber A. Richter M, et al. Hematol Oncol Clin North Am. 2017 Oct;31(5):771-785. doi: 10.1016/j.hoc.2017.06.001. Hematol Oncol Clin North Am. 2017. PMID: 28895846 Free PMC article. Review.
Cited by
- RNA-mediated gene silencing in hematopoietic cells.
Venturini L, Eder M, Scherr M. Venturini L, et al. J Biomed Biotechnol. 2006;2006(4):87340. doi: 10.1155/JBB/2006/87340. J Biomed Biotechnol. 2006. PMID: 17057372 Free PMC article. - Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent.
Sutton RE, Reitsma MJ, Uchida N, Brown PO. Sutton RE, et al. J Virol. 1999 May;73(5):3649-60. doi: 10.1128/JVI.73.5.3649-3660.1999. J Virol. 1999. PMID: 10196257 Free PMC article. - Genome Modification Technologies and Their Applications in Avian Species.
Lee HJ, Kim YM, Ono T, Han JY. Lee HJ, et al. Int J Mol Sci. 2017 Oct 26;18(11):2245. doi: 10.3390/ijms18112245. Int J Mol Sci. 2017. PMID: 29072628 Free PMC article. Review. - Pseudotyped Viruses.
Wang Y, Zhou Z, Wu X, Li T, Wu J, Cai M, Nie J, Wang W, Cui Z. Wang Y, et al. Adv Exp Med Biol. 2023;1407:1-27. doi: 10.1007/978-981-99-0113-5_1. Adv Exp Med Biol. 2023. PMID: 36920689 - Lentivirus vector gene expression during ES cell-derived hematopoietic development in vitro.
Hamaguchi I, Woods NB, Panagopoulos I, Andersson E, Mikkola H, Fahlman C, Zufferey R, Carlsson L, Trono D, Karlsson S. Hamaguchi I, et al. J Virol. 2000 Nov;74(22):10778-84. doi: 10.1128/jvi.74.22.10778-10784.2000. J Virol. 2000. PMID: 11044122 Free PMC article.
References
- Morrison S J, Weissman I L. Immunity. 1994;1:661–673. - PubMed
- Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. - PubMed
- Nibley W E, Spangrude G J. Bone Marrow Transplant. 1998;21:345–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical