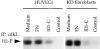Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression - PubMed (original) (raw)
Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression
S Bourdoulous et al. J Cell Biol. 1998.
Abstract
Adherent cells assemble fibronectin into a fibrillar matrix on their apical surface. The fibril formation is initiated by fibronectin binding to the integrins alpha5 beta1 and alphav beta3, and is completed by a process that includes fibronectin self-assembly. We found that a 76- amino acid fragment of fibronectin (III1-C) that forms one of the self-assembly sites caused disassembly of preformed fibronectin matrix without affecting cell adhesion. Treating attached fibroblasts or endothelial cells with III1-C inhibited cell migration and proliferation. Rho-dependent stress fiber formation and Rho-dependent focal contact protein phosphorylation were also inhibited, whereas Cdc42 was activated, leading to actin polymerization into filopodia. ACK (activated Cdc42-binding kinase) and p38 MAPK (mitogen-activated protein kinase), two downstream effectors of Cdc42, were activated, whereas PAK (p21-activated kinase) and JNK/SAPK (c-Jun NH2-terminal kinase/ stress-activated protein kinase) were inhibited. III1-C treatment also modulated activation of JNK and ERK (extracellular signal-regulated kinases) in response to growth factors, and reduced the activity of the cyclin E-cdk2 complex. These results indicate that the absence of fibronectin matrix causes activation of Cdc42, and that fibronectin matrix is required for Rho activation and cell cycle progression.
Figures
Figure 1
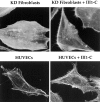
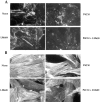
Treatment with III1-C removes surface fibronectin matrix. Immunofluorescence staining of fibronectin: (A, top) on the surface of HUVECs with or without treatment with 20 μM III1-C or with 20 μM of the control fragment III-CF for 4 h in serum-free medium, or (A, bottom) on the surface KD fibroblasts after treatment with or without 20 μM III1-C for 16 h in serum-free medium; (B) on the surface of KD fibroblasts treated with the indicated concentrations of III1-C for 16 h; (C) in Triton X-100– permeabilized KD fibroblasts after treatment for 16 h with 20 μM III1-C in serum free-medium or with the medium alone.
Figure 1
Treatment with III1-C removes surface fibronectin matrix. Immunofluorescence staining of fibronectin: (A, top) on the surface of HUVECs with or without treatment with 20 μM III1-C or with 20 μM of the control fragment III-CF for 4 h in serum-free medium, or (A, bottom) on the surface KD fibroblasts after treatment with or without 20 μM III1-C for 16 h in serum-free medium; (B) on the surface of KD fibroblasts treated with the indicated concentrations of III1-C for 16 h; (C) in Triton X-100– permeabilized KD fibroblasts after treatment for 16 h with 20 μM III1-C in serum free-medium or with the medium alone.
Figure 2
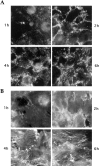
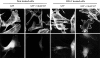
III1-C treatment stimulates formation of filopodial protrusions of the cytoskeleton at the cell periphery. Rhodamine-phalloidin staining of the actin cytoskeleton (top) in KD fibroblasts after treatment with or without 20 μM III1-C for 16 h in serum free medium; (bottom) in HUVECs with or without treatment with 20 μM III1-C for 4 h in serum-free medium. The images were produced with a confocal microscope.
Figure 3
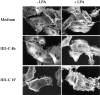
Fibronectin matrix removal blocks Rho-mediated stress fiber formation. Rhodamine-phalloidin staining of the actin cytoskeleton. HUVECs were treated in serum-free medium in the absence (medium, top) or presence of 20 μM III1-C for 4 h (middle) or 20 μM III1-C for the last 15 min. The cells were then stimulated (right) or left unstimulated (left) with 5 μM LPA for 15 min. The images were produced with a confocal microscope.
Figure 4
Fibronectin matrix removal blocks Rho-mediated tyrosine phosphorylation of focal contact proteins FAK and paxillin. (A) HUVECs were treated for 4 h with 20 μM III1-C in serum-free medium to remove fibronectin matrix (+) or with the medium alone (−). Where indicated, LPA cells were stimulated with 5 μM LPA for 15 min before lysis. As a control for the effects of III1-C without removing fibronectin matrix, 20 μM III1-C was added for 15 min in absence (III1-C) or presence of 5 μM LPA (III1-C + LPA). FAK or paxillin were immunoprecipitated and immunoblotted with an anti-phosphotyrosine antibody (PY20). The blots were reprobed with anti-FAK or anti-paxillin antibodies to show that similar protein levels were immunoprecipitated and to detect FAK that had coimmunoprecipitated with paxillin. (B) Suspended HUVECs were treated for 1 h with (+) or without (−) 20 μM III1-C in serum-free medium before plating on dishes coated with 10 μg/ml fibronectin, vitronectin, or laminin. The cells were lysed after 30 min, and FAK or paxillin was immunoprecipitated and immunoblotted with an anti-phosphotyrosine antibody (PY20). The experiments were carried out three to five times, and a representative result is shown.
Figure 5
Time course of fibronectin matrix deposition and stress fiber organization after attachment of cells on substrate. Trypsinized KD fibroblasts were plated on a mixture of collagen IV and vitronectin in serum-free medium and stained after 1, 2, 4, or 6 h for cell surface fibronectin (A) or actin cytoskeleton (B).
Figure 6
Inhibition of fibronectin matrix assembly with function-blocking integrin antibodies prevents stress fiber formation. Trypsinized KD fibroblasts were plated on a mixture of collagen IV and vitronectin in serum-free medium. The cells were allowed to attach and spread for 1 h before adding function-blocking antibodies directed against the β1 (P4C10, 10 μg/ml) or αvβ3 (LM609, 10 μg/ml) integrins individually or in combination. After an additional 6 h, the cells were stained for cell surface fibronectin (A) or actin cytoskeleton (B). (C) HUVECs were grown on a mixture of fibronectin and laminin in complete medium for 48 h, and were then treated with the function-blocking antibodies directed against the α5 (P1D6, 10 μg/ml) or αvβ3 (LM609, 10 μg/ml) integrins individually or in combination in serum-free medium. The cells were stained after 4 h for actin cytoskeleton.
Figure 6
Inhibition of fibronectin matrix assembly with function-blocking integrin antibodies prevents stress fiber formation. Trypsinized KD fibroblasts were plated on a mixture of collagen IV and vitronectin in serum-free medium. The cells were allowed to attach and spread for 1 h before adding function-blocking antibodies directed against the β1 (P4C10, 10 μg/ml) or αvβ3 (LM609, 10 μg/ml) integrins individually or in combination. After an additional 6 h, the cells were stained for cell surface fibronectin (A) or actin cytoskeleton (B). (C) HUVECs were grown on a mixture of fibronectin and laminin in complete medium for 48 h, and were then treated with the function-blocking antibodies directed against the α5 (P1D6, 10 μg/ml) or αvβ3 (LM609, 10 μg/ml) integrins individually or in combination in serum-free medium. The cells were stained after 4 h for actin cytoskeleton.
Figure 7
Expression of a dominant negative form of Cdc42 prevents the formation of III1-C–induced filopodia. HUVECs cells were transfected GFP alone, or with GFP and Cdc42N17 before treatment with 20 μM III1-C for 4 h in serum-free medium and stained for actin cytoskeleton (top). In the lower panel, the same cells were analyzed for GFP expression to identify the transfected cells.
Figure 8
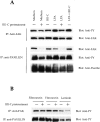
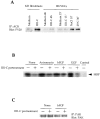
Tyrosine phosphorylation of ACK and inhibition of PAK1 kinase activity in response to fibronectin matrix removal by III1-C. (A) Tyrosine phosphorylation of ACK: KD fibroblasts were treated with 20 μM III1-C for 16 h. HUVECs were treated with 20 μM III1-C for 4 h or 15 min, or were stimulated by hyperosmotic shock (0.8 M NaCl for 10 min) or by decreasing the temperature (25°C for 30 min). Serum-free medium was used as a control (medium). ACK was immunoprecipitated and immunoblotted with an anti-phosphotyrosine antibody (PY20). (B) PAK1 in vitro kinase assay using MBP as the substrate. HUVECs were pretreated with 20 μM III1-C for 4 h in serum-free medium to remove fibronectin matrix (+), or were treated with the medium alone (−), and where indicated, were stimulated by anisomycin (10 μg/ml), bFGF (10 ng/ml), or EGF (10 ng/ml) for 5 min before lysis. This experiment was carried out four times with similar results, and representative results are shown. (C) Controls confirmed that an equivalent amount of PAK protein was immunoprecipitated from the III1-C treated and control cells.
Figure 9
Fibronectin matrix removal activates p38 MAPK. (A) Immunoblotting with a phosphospecific (Tyr182) p38 antibody or conventional p38 antibody. HUVECs were pretreated (+) with 20 μM III1-C for 4 h in serum-free medium to remove fibronectin matrix, or were left untreated (−). Where indicated, the cells were then stimulated with 5 μM LPA for 15 min before lysis (LPA). As a control, HUVECs were treated with 20 μM III1-C for the final 15 min in the absence (III1-C) or the presence of 5 μM LPA (III1-C + LPA). Alternatively, cells were stimulated with 10 ng/ml EGF or bFGF for 15 min before lysis. Treatment with serum-free medium was used as a control (Medium) in both experiments. (B) P38 MAPK in vitro kinase assay using ATF-2 as substrate. HUVECs were treated (+) with 20 μM III1-C for 4 h and, where indicated, were stimulated with bFGF (10 ng/ml) or anisomycin (10 μg/ml) for 15 min or left untreated in serum-free medium (−) and then lysed.
Figure 10
Fibronectin matrix removal inhibits the activity of the cyclin E–cdk2 complex. Synchronized HUVECs and KD fibroblasts were treated with 10 μM III1-C or 10 μg/ml fibronectin (FN) as a control for 18 h in complete medium. Cdk2 was immunoprecipitated and in vitro kinase assays for the cyclin E–cdk2 complex were performed using histone-1 as the substrate. In vitro kinase assays were also performed on lysates that were immunoprecipitated with an unrelated antibody as a control (Control).
Similar articles
- An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1.
Olson MF, Ashworth A, Hall A. Olson MF, et al. Science. 1995 Sep 1;269(5228):1270-2. doi: 10.1126/science.7652575. Science. 1995. PMID: 7652575 - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review. - The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway.
Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. Coso OA, et al. Cell. 1995 Jun 30;81(7):1137-46. doi: 10.1016/s0092-8674(05)80018-2. Cell. 1995. PMID: 7600581 - Actin cytoskeleton organization in response to integrin-mediated adhesion.
Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G. Defilippi P, et al. Microsc Res Tech. 1999 Oct 1;47(1):67-78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10506763 Review.
Cited by
- Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42.
Adams JC, Schwartz MA. Adams JC, et al. J Cell Biol. 2000 Aug 21;150(4):807-22. doi: 10.1083/jcb.150.4.807. J Cell Biol. 2000. PMID: 10953005 Free PMC article. - Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis.
Thomas D, Radhakrishnan P. Thomas D, et al. Mol Cancer. 2019 Jan 21;18(1):14. doi: 10.1186/s12943-018-0927-5. Mol Cancer. 2019. PMID: 30665410 Free PMC article. Review. - The Borgs, a new family of Cdc42 and TC10 GTPase-interacting proteins.
Joberty G, Perlungher RR, Macara IG. Joberty G, et al. Mol Cell Biol. 1999 Oct;19(10):6585-97. doi: 10.1128/MCB.19.10.6585. Mol Cell Biol. 1999. PMID: 10490598 Free PMC article. - Chimeric fibronectin matrix mimetic as a functional growth- and migration-promoting adhesive substrate.
Roy DC, Wilke-Mounts SJ, Hocking DC. Roy DC, et al. Biomaterials. 2011 Mar;32(8):2077-87. doi: 10.1016/j.biomaterials.2010.11.050. Epub 2010 Dec 24. Biomaterials. 2011. PMID: 21185596 Free PMC article.
References
- Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecules. J Biol Chem. 1994;269:27863–27868. - PubMed
- Assoian RK, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. - PubMed
- Busk M, Pytela R, Sheppard D. Characterization of the integrin alphavbeta6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–5796. - PubMed
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA67224/CA/NCI NIH HHS/United States
- CA30199/CA/NCI NIH HHS/United States
- P30 CA030199/CA/NCI NIH HHS/United States
- CA62042/CA/NCI NIH HHS/United States
- F32 CA067424/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous