Identification of regulators for Ypt1 GTPase nucleotide cycling - PubMed (original) (raw)
Identification of regulators for Ypt1 GTPase nucleotide cycling
S Jones et al. Mol Biol Cell. 1998 Oct.
Free PMC article
Abstract
Small GTPases of the Ypt/Rab family are involved in the regulation of vesicular transport. Cycling between the GDP- and GTP-bound forms and the accessory proteins that regulate this cycling are thought to be crucial for Ypt/Rab function. Guanine nucleotide exchange factors (GEFs) stimulate both GDP loss and GTP uptake, and GTPase-activating proteins (GAPs) stimulate GTP hydrolysis. Little is known about GEFs and GAPs for Ypt/Rab proteins. In this article we report the identification and initial characterization of two factors that regulate nucleotide cycling by Ypt1p, which is essential for the first two steps of the yeast secretory pathway. The Ypt1p-GEF stimulates GDP release and GTP uptake at least 10-fold and is specific for Ypt1p. Partially purified Ypt1p-GEF can rescue the inhibition caused by the dominant-negative Ypt1p-D124N mutant of in vitro endoplasmic reticulum-to-Golgi transport. This mutant probably blocks transport by inhibiting the GEF, suggesting that we have identified the physiological GEF for Ypt1p. The Ypt1p-GAP stimulates GTP hydrolysis by Ypt1p up to 54-fold, has a higher affinity for the GTP-bound form of Ypt1p than for the GDP-bound form, and is specific to a subgroup of exocytic Ypt proteins. The Ypt1p-GAP activity is not affected by deletion of two genes that encode known Ypt GAPs, GYP7 and GYP1, nor is it influenced by mutations in SEC18, SEC17, or SEC22, genes whose products are involved in vesicle fusion. The GEF and GAP activities for Ypt1p localize to particulate cellular fractions. However, contrary to the predictions of current models, the GEF activity localizes to the fraction that functions as the acceptor in an endoplasmic reticulum-to-Golgi transport assay, whereas the GAP activity cofractionates with markers for the donor. On the basis of our current and previous results, we propose a new model for the role of Ypt/Rab nucleotide cycling and the factors that regulate this process.
Figures
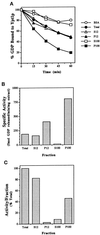
Figure 1
Identification of a GDP release-stimulating activity for Ypt1p in the P100 fraction of yeast cells. (A) Time course of GDP release from Ypt1p in the presence of fractionated yeast extracts is shown. Ypt1p-[3H]GDP was incubated in the presence of cell extract or BSA at 1 mg/ml. Values represent the percent of label retained at the indicated time, relative to that at the beginning of the incubation. Fractions are the total extract (closed circles), S12 (open triangles), P12 (closed triangles), S100 (open squares), P100 (closed squares), and no extract (BSA; open circles). (B) Ypt1p-GEF is enriched in the P100 fraction. To determine the specific activity of GDP release in the different fractions, we varied the fraction concentration from 0.2 to 1 mg/ml in the GDP release reaction and withdrew samples at 3 min intervals over 15 min. Conditions were selected under which protein and time were limiting, and rates of GDP release were determined from the linear portion of the curve. (C) The P100 fraction contains approximately one-half of the Ypt1p-GEF activity in the total extract. The portion of activity per fraction was calculated as the product of the specific activity (Figure 1B) and amount (milligrams) of protein in the fractions obtained from ∼5000 OD600 units of cells. Results shown in this figure are representative of at least three experiments, and values from similar experiments agreed to within 5%.
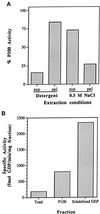
Figure 2
Association of the Ypt1p-GEF with the P100 fraction and preparation of the solubilized GEF fraction. (A) Extraction of Ypt1p-GEF from the P100 fraction by salt but not by detergents is shown. P100 was treated with 1% Triton X-100, 30 mM _n_-octylglucoside, or 0.5 M NaCl. Equal volumes of the P100 fraction and the treated pellets and supernatants were diluted at least fivefold into the reaction mixture (NaCl concentrations were at or below 100 mM, a concentration that does not affect intrinsic or stimulated exchange) and were assayed for their ability to stimulate release of [3H]GDP from Ypt1p as described. Addition of 1% Triton X-100, 30 mM _n_-octylglucoside, or 0.5 M NaCl to the unfractionated P100 had no effect on its activity. Extracts were prepared at least twice and were assayed at least twice per extraction with equivalent results. Extraction with 1% Triton X-100 and 30 mM _n_-octylglucoside gave identical results and are presented as Detergent. (B) Sequential extraction of the P100 fraction with Triton X-100 and NaCl yields a soluble Ypt1p-GEF activity and an additional fourfold increase in specific activity. The solubilized GEF fraction is the S100 fraction from sequential extraction of the P100 fraction with 1% Triton X-100, followed by extraction of the resulting pellet with 0.5 M NaCl. The total enrichment of the Ypt1p-GEF activity in the solubilized fraction relative to the crude cell extract is 16- to 20-fold. Similar results were obtained with four independent preparations.
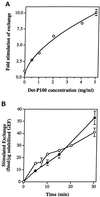
Figure 3
Stimulation of GTP uptake by Ypt1p-GEF. (A) Increasing concentrations of the Det-P100 (Triton X-100–extracted) fraction result in increasing stimulation of GTP uptake by Ypt1p. Ten picomoles of Ypt1p preloaded with nonradioactive GDP were incubated with varying amounts of the Det-P100 fraction in reaction mixtures containing [α-32P]GTP. Reactions were performed for 15 min, and the amount of isotope bound to Ypt1p was determined by immunoprecipitation of samples removed at 2–5 min intervals. The results represent the averages of two independent experiments. Error bars represent SEM. (B) Partially purified solubilized Ypt1p-GEF stimulates GTP uptake as well as GDP release. Ten picomoles of Ypt1p preloaded with either nonradioactive or [3H]GDP were incubated with varying amounts of the solubilized GEF fraction in reaction mixtures containing [α-32P]GTP or nonradioactive GTP, respectively. Samples were taken at intervals, and the amount of isotope bound to Ypt1p was determined by immunoprecipitation ([32P]GTP uptake assay; closed circles) or nitrocellulose filtration ([3H]GDP release assay; open circles). Intrinsic rates for GDP release and GTP uptake, measured in the presence of BSA or ovalbumin, respectively, were subtracted. The results represent the averages of two to nine determinations per point. Error bars represent SEM.
Figure 4
The Ypt1p-GEF activity present in the Det-P100 (Triton X-100– or _n_-octylglucoside–extracted) fraction does not act on Ypt32p or Sec4p and is different from the exchange factor for Ras2p. Ten picomoles of Ypt1p-[3H]GDP (A), Ypt32p-[3H]GDP (B), Sec4p-[3H]GDP (C), or Ras2p-[3H]GDP (D) were incubated in the presence of the Det-P100 fraction (closed circles; 5 mg/ml, except 2 mg/ml Ypt32p) or BSA (open circles), and the stimulated and intrinsic rates of [3H]GDP release were determined by sampling at the times indicated. Including the Det-P100 fraction at 1 mg/ml resulted in ∼6-fold stimulation of GDP release from Ypt1p (our unpublished observations), but no stimulation was observed for Ypt32p at concentrations of Det-P100 up to 2 mg/ml. Including 5 mg/ml of Det-P100 fraction resulted in ∼12-fold stimulation of GDP-release from Ypt1p, no stimulation for Sec4, and approximately ∼4- to 6-fold stimulation for Ras2p. The stimulated GDP release by Ypt1p can be completely inhibited by Ypt1-D124N dominant-mutant protein (1.5 μM). However, the GEF activity for Ras2p present in this fraction is not affected by this Ypt1 mutant protein (A and D; open triangles). The results represent the averages of two experiments with duplicates for each time point. Error bars represent SEM.
Figure 5
Partial purification of the Ypt1p-GEF. (A) Gel filtration column. Two partially overlapping Ypt1p-GEF peaks are collected into distinct pools. The solubilized GEF fraction (Det-P100 extracted by salt) was separated on a Sephacryl S-300 HR column. Stimulated GDP loss at 30 min is graphed versus fraction number (squares). Protein concentration in the fractions as determined by absorbance at 280 nm is plotted (solid black line). The inverted triangles at the top show the positions of ferritin (450 kDa), alcohol dehydrogenase (150 kDa), ovalbumin (45 kDa), and myoglobin (17.8 kDa). Pool A and Pool B were collected separately for further analysis and purified and concentrated as described in MATERIALS AND METHODS. The chromatogram represents the average of three independent experiments performed under identical conditions. (B) HAP column. The S-300 A and S-300 B pools generate single, distinct peaks of Ypt1p-GEF activity on the hydroxyapatite column. The S-300 pool A and the S-300 pool B were loaded onto separate ceramic hydroxyapatite columns and eluted with a phosphate gradient. The results shown are the stimulated GDP loss at 30 min in fractions from the HAP column loaded with the S-300 A pool (squares), the absorbance at 280 nm of the HAP column loaded with the S-300 A pool (solid line), the stimulated GDP loss at 30 min in fractions from the HAP column loaded with the S-300 B pool (circles), and the absorbance at 280 nm of the HAP column loaded with the S-300 B pool (black dotted line). The phosphate gradient (from 10 to 200 mM) is indicated by the diagonal dotted line. Fractions with significant guanine nucleotide exchange activity (filled symbols) were combined into HAP A or HAP B pools (indicated by the inverted triangles at the top). The results represent two independent experiments.
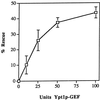
Figure 6
Inhibition by Ypt1p-D124N of the in vitro transport reaction is relieved by Ypt1p-GEF, HAP peak A. The indicated amounts of exchange activity from HAP Pool A (1 unit is defined as 1 fmol of GDP released from 0.1 μM Ypt1p per minute per milligram of GEF) were mixed with 1.1 μg of Ypt1p-D124N protein (2 μl) in a total volume of 38 μl of Buffer 88 and were incubated on ice for 20 min. This amount of Ypt1p-D124N protein inhibits the ER-to-Golgi transport reaction 50% under these conditions. The S1 cell fraction (2 μl, ∼100 μg) was added to each reaction, and then incubations were continued on ice for 20 more minutes before the reactions were initiated by the addition of 10 μl of permeabilized yeast cells preloaded with 35S-α-factor. The reactions were incubated at 20°C for 90 min. The percent rescue of inhibition of transport was calculated by setting 0% inhibition equal to the amount of transport in the uninhibited control reaction and by setting 100% inhibition equal to the amount of ER-to-Golgi transport in the fully inhibited reaction (without HAP Pool A). This figure represents the average of two independent experiments with each sample measured as duplicates, and the error bars represent the SD of all four measurements for each point.
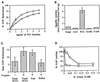
Figure 7
Identification of a Ypt1p-GAP activity in the P12 cellular fraction and its solubilization. (A) GAP activity is linear with P12 concentration. Ypt1p (2 nM) preloaded with [γ-32P]GTP was incubated with the indicated quantities of P12 fraction at 30°C. GTP hydrolysis was measured by the charcoal-binding assay. Squares represent a 15 min incubation; circles represent a 30 min incubation. Data are expressed as the percent of the total 32P-labeled pool of GTP bound to Ypt1p that was hydrolyzed, and the intrinsic rate of GTP hydrolysis by Ypt1p was subtracted. Results are the average of two independent experiments. Error bars represent the range divided by 2. (B) Localization of GAP activity to P12 (specific activity) is shown. Crude lysates (Total) were generated from GPY60 cells. Lysates were centrifuged at 12,000 × g to generate S12 and P12. The S12 fraction was further centrifuged at 100,000 × g to generate S100 and P100. Ypt1p (2 nM; 200 fmol) preloaded with [γ-32P]GTP was incubated with the indicated cell fractions at 0.5 mg/ml for 15 min at 30°C. GTP hydrolysis was measured as described above. Specific activity is a measure of the femtomoles of GTP hydrolyzed by Ypt1p per minute per microgram of added cell fraction. Results are the average of three independent measurements performed with cell fractions from two independent fractionations. Error bars represent the SEM. (C) GAP activity is stimulated and extracted by limited trypsin digestion. Seven hundred micrograms of a P12 fraction were left untreated (Untreated Total) or treated with trypsin at 0.1 mg/ml on ice for 1 h after which time trypsin inhibitor at 0.2 mg/ml was added (Treated Total). The trypsin-treated sample was then centrifuged at 12,000 × g for 10 min to generate supernatant (Treated Sup) and pellet (Treated Pellet) fractions. The pellet was resuspended to the original volume in Buffer 88 before centrifugation. An equal volume of each sample was assayed for GAP activity as described above. Including trypsin inhibitor at the beginning of the incubation completely prevents extraction and stimulation of the GAP (our unpublished observations). Results are the average of three independent measurements. Error bars represent the SEM. (D) GAP activity is inhibited by Triton X-100. Inhibition of GAP activity in the P12 (0.5 mg/ml; squares) or in the trypsin-solubilized GAP (extracted as described in C from 0.05 mg of P12; circles) by Triton X-100 was tested. GAP assays were performed as described above in the presence of the indicated final concentrations of Triton X-100 (vol/vol) for 15 min at 30°C. Data expressed as the percent of GAP activity from the uninhibited reaction are typical of two independent experiments.
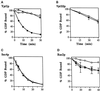
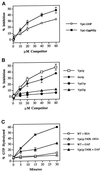
Figure 8
Substrate specificity of Ypt1p-GAP. (A) Ypt1 GAP has a higher affinity for the GTP form than for the GDP form of Ypt1p. Ypt1p (2 nM) preloaded with [γ-32P]GTP was mixed with the indicated concentrations of competitor Ypt1p preloaded with cold GppNHp (squares) or GDP (circles). P12 was added to a concentration of 0.75 mg/ml as a source for GAP activity. Reactions contained 1 mM each ATP, GTP, and GDP. Incubations were performed for 30 min at 30°C. The decrease in the rate of GTP hydrolysis observed is expressed as the percent inhibition of the rate determined for the uninhibited control (0 μM competitor). Data are the average of two experiments. Error bars represent SEM. (B) Competition by other exocytic Ypt proteins for Ypt1p-GAP is shown. To determine which proteins Ypt1p-GAP can interact with, we assayed various Ypt proteins for their ability to compete with Ypt1p for the Ypt1p-GAP. Reactions were performed as described in A in the presence of the indicated concentrations of Ypt1p (squares), Sec4p (diamonds), Ypt32p (triangles), or Ypt31p (circles) preloaded with GppNHp. Data are the average of two experiments. Error bars represent SEM. (C) Ypt1p-T40K is defective in GAP-stimulated GTP hydrolysis. Wild-type (squares) and Ypt1p-T40K (triangles) proteins were preloaded with [α-32P]GTP for 15 min at 30°C. GTP hydrolysis assays were performed by incubating 2 nM preloaded Ypt1p with 1.5 mg/ml P12 (GAP-stimulated hydrolysis; filled symbols) or without the P12 fraction (intrinsic hydrolysis; open symbols) at 30°C. Aliquots were removed at the indicated time points, GTP hydrolysis was determined by TLC, and radioactivity was detected with a radioanalytic imager. Hydrolysis is expressed as the percent of GDP detected divided by the total nucleotide detected (GDP + GTP). Results are the average of two independent measurements. Error bars represent the range divided by 2.
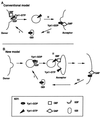
Figure 9
Two models for the role of nucleotide cycling and factors that regulate it in Ypt/Rab-mediated vesicular transport, using Ypt1p as an example. (A) Conventional model (Goud and McCaffrey, 1991; Novick and Brennwald, 1993). In addition to GEFs and GAPs, two other factors that influence nucleotide cycling of Ypt/Rab proteins are GDI and GDI-dissociation factor (GDF). GDI is implicated in the recycling of Ypt/Rab proteins between membranes (Araki et al. 1990; Soldati et al., 1993), and GDFs are thought to function as receptors or chaperones for Ypt/Rab proteins (Dirac-Svejstrup et al., 1997). Step I, recruitment of Ypt1p-GDP to the donor membrane by GDF and nucleotide exchange by GEF to yield Ypt1p-GTP are shown; Ypt1p-GTP is present on forming secretory vesicles. Step II, GTP hydrolysis is required or is coupled with fusion of secretory vesicles with the acceptor compartment. Step III, GDI recycles Ypt1p-GDP back to the donor membrane. (B) New model, based on this article and our previous work (Jones et al., 1995; Richardson et al., 1998). Step I, Ypt1p-GDP is recruited to the vesicle (or the donor membrane) by GDF. Step II, nucleotide exchange by GEF is coupled to vesicle fusion with the acceptor compartment. Step III, GTP hydrolysis occurs late in the pathway to generate Ypt1p-GDP, and GDI recycles Ypt1p-GDP for the next cycle. The important features that distinguish this model from the conventional model are the major role suggested for nucleotide exchange and the factor that mediates it (GEF), the minor role of GTP hydrolysis and GAP not in vesicle fusion but in Ypt1p recycling, and the suggested localization of these regulators. If GAP localizes to the plasma membrane, as shown here, it might have a role in GDI-mediated Ypt/Rab protein recycling (which is not required for Ypt1p function). In ypt1-Q67L mutant cells, when GTP hydrolysis is defective, Ypt1p-GTP might be recycled via a GDI-independent mechanism. If GAP localizes to the ER, it might be there to stimulate GTP hydrolysis by Ypt1p to allow better interaction with the GEF in the next cycle (see DISCUSSION).
Similar articles
- GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport.
Richardson CJ, Jones S, Litt RJ, Segev N. Richardson CJ, et al. Mol Cell Biol. 1998 Feb;18(2):827-38. doi: 10.1128/MCB.18.2.827. Mol Cell Biol. 1998. PMID: 9447979 Free PMC article. - Significance of GTP hydrolysis in Ypt1p-regulated endoplasmic reticulum to Golgi transport revealed by the analysis of two novel Ypt1-GAPs.
De Antoni A, Schmitzová J, Trepte HH, Gallwitz D, Albert S. De Antoni A, et al. J Biol Chem. 2002 Oct 25;277(43):41023-31. doi: 10.1074/jbc.M205783200. Epub 2002 Aug 19. J Biol Chem. 2002. PMID: 12189143 - Requirement of nucleotide exchange factor for Ypt1 GTPase mediated protein transport.
Jones S, Litt RJ, Richardson CJ, Segev N. Jones S, et al. J Cell Biol. 1995 Sep;130(5):1051-61. doi: 10.1083/jcb.130.5.1051. J Cell Biol. 1995. PMID: 7657691 Free PMC article. - Small GTP-binding proteins and their role in transport.
Goud B, McCaffrey M. Goud B, et al. Curr Opin Cell Biol. 1991 Aug;3(4):626-33. doi: 10.1016/0955-0674(91)90033-u. Curr Opin Cell Biol. 1991. PMID: 1663370 Review. No abstract available. - The role of ADP-ribosylation factor and SAR1 in vesicular trafficking in plants.
Memon AR. Memon AR. Biochim Biophys Acta. 2004 Jul 1;1664(1):9-30. doi: 10.1016/j.bbamem.2004.04.005. Biochim Biophys Acta. 2004. PMID: 15238254 Review.
Cited by
- The TRAPP complex: insights into its architecture and function.
Sacher M, Kim YG, Lavie A, Oh BH, Segev N. Sacher M, et al. Traffic. 2008 Dec;9(12):2032-42. doi: 10.1111/j.1600-0854.2008.00833.x. Epub 2008 Oct 14. Traffic. 2008. PMID: 18801063 Free PMC article. Review. - Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p.
Siniossoglou S, Peak-Chew SY, Pelham HR. Siniossoglou S, et al. EMBO J. 2000 Sep 15;19(18):4885-94. doi: 10.1093/emboj/19.18.4885. EMBO J. 2000. PMID: 10990452 Free PMC article. - Newer Methods Drive Recent Insights into Rab GTPase Biology: An Overview.
Li G, Segev N. Li G, et al. Methods Mol Biol. 2021;2293:1-18. doi: 10.1007/978-1-0716-1346-7_1. Methods Mol Biol. 2021. PMID: 34453706 - Trs20 is required for TRAPP II assembly.
Taussig D, Lipatova Z, Kim JJ, Zhang X, Segev N. Taussig D, et al. Traffic. 2013 Jun;14(6):678-90. doi: 10.1111/tra.12065. Epub 2013 Mar 25. Traffic. 2013. PMID: 23465091 Free PMC article. - Ypt/Rab GTPases: principles learned from yeast.
Lipatova Z, Hain AU, Nazarko VY, Segev N. Lipatova Z, et al. Crit Rev Biochem Mol Biol. 2015;50(3):203-11. doi: 10.3109/10409238.2015.1014023. Epub 2015 Feb 23. Crit Rev Biochem Mol Biol. 2015. PMID: 25702751 Free PMC article. Review.
References
- Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990;256:13007–13015. - PubMed
- Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. - PubMed
- Balch WE. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990;15:473–477. - PubMed
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HD007009/HD/NICHD NIH HHS/United States
- 5T32 GM-07151-20/GM/NIGMS NIH HHS/United States
- 5T32 HL-07381/HL/NHLBI NIH HHS/United States
- T32 HL007381/HL/NHLBI NIH HHS/United States
- R01 GM045444/GM/NIGMS NIH HHS/United States
- 5T32 HD-07009/HD/NICHD NIH HHS/United States
- T32 GM007151/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous